Question: Im pretty sure I am setting this up wrong, can you tell me where I am messing up? (1c): What is the theoretical yield of

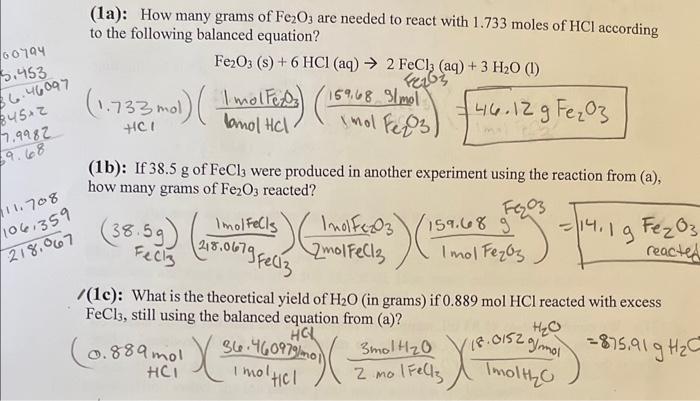
(1c): What is the theoretical yield of H2O (in grams) if 0.889molHCl reacted with excess FeCl3, still using the balanced equation from (a)? (1a): How many grams of Fe2O3 are needed to react with 1.733 moles of HCl according to the following balanced equation? Fe2O3(s)+6HCl(aq)2FeCl3(aq)+3H2O(l) (1b): If 38.5g of FeCl3 were produced in another experiment using the reaction from (a), how many grams of Fe2O3 reacted? (38.5g)FeCl3(218.067FeCl31molFeCl3)(2molFeCl3lmolFe2F3)(1molFe2F3159.68g2F3)=14.1Fe20 (1c): What is the theoretical yield of H2O (in grams) if 0.889molHCl reacted with excess FeCl3, still using the balanced equation from (a)? (0.889molHClHCl)(1molHCl36.460979molmol)(2molFeCl33mol2O)(1molH2O(8.0152gmol)=875.91gH2O
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


