Question: im trying to check my work for determination 1 from A to E. the concentration of Na2s2O3 is 0.01935 M and the volune of the
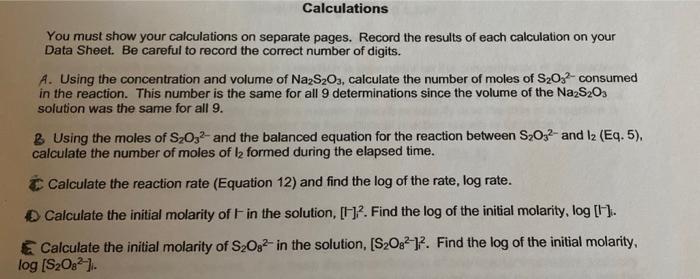



Calculations You must show your calculations on separate pages. Record the results of each calculation on your Data Sheet. Be careful to record the correct number of digits. A. Using the concentration and volume of Na2S2O3, calculate the number of moles of S2022-consumed in the reaction. This number is the same for all 9 determinations since the volume of the NazS203 solution was the same for all 9. B Using the moles of S2O32- and the balanced equation for the reaction between S2O32- and 12 (Eq.5), calculate the number of moles of la formed during the elapsed time. Calculate the reaction rate (Equation 12) and find the log of the rate, log rate. Calculate the initial molarity of Fin the solution, [2. Find the log of the initial molarity, log (1). Calculate the initial molarity of S2O32- in the solution, [S2082-)2. Find the log of the initial molarity, log [S2082) Determination 1 2 3 5 on 311 229 175 138 112 time elapsed for color change, s 2 S2O32- (aq) + 12 (aq) 2 (aq) + S,062 (aq) fast (Eq. 5) number of moles ofl, formed, mol elapsed time, s (Eq. 12) reaction rate, mol 12/s
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


