Question: Procedure You will perform four separate trials, each involving a different solution. PREPARE AND WORK WITH ONE SOLUTION AT A TIME. The reaction is carried

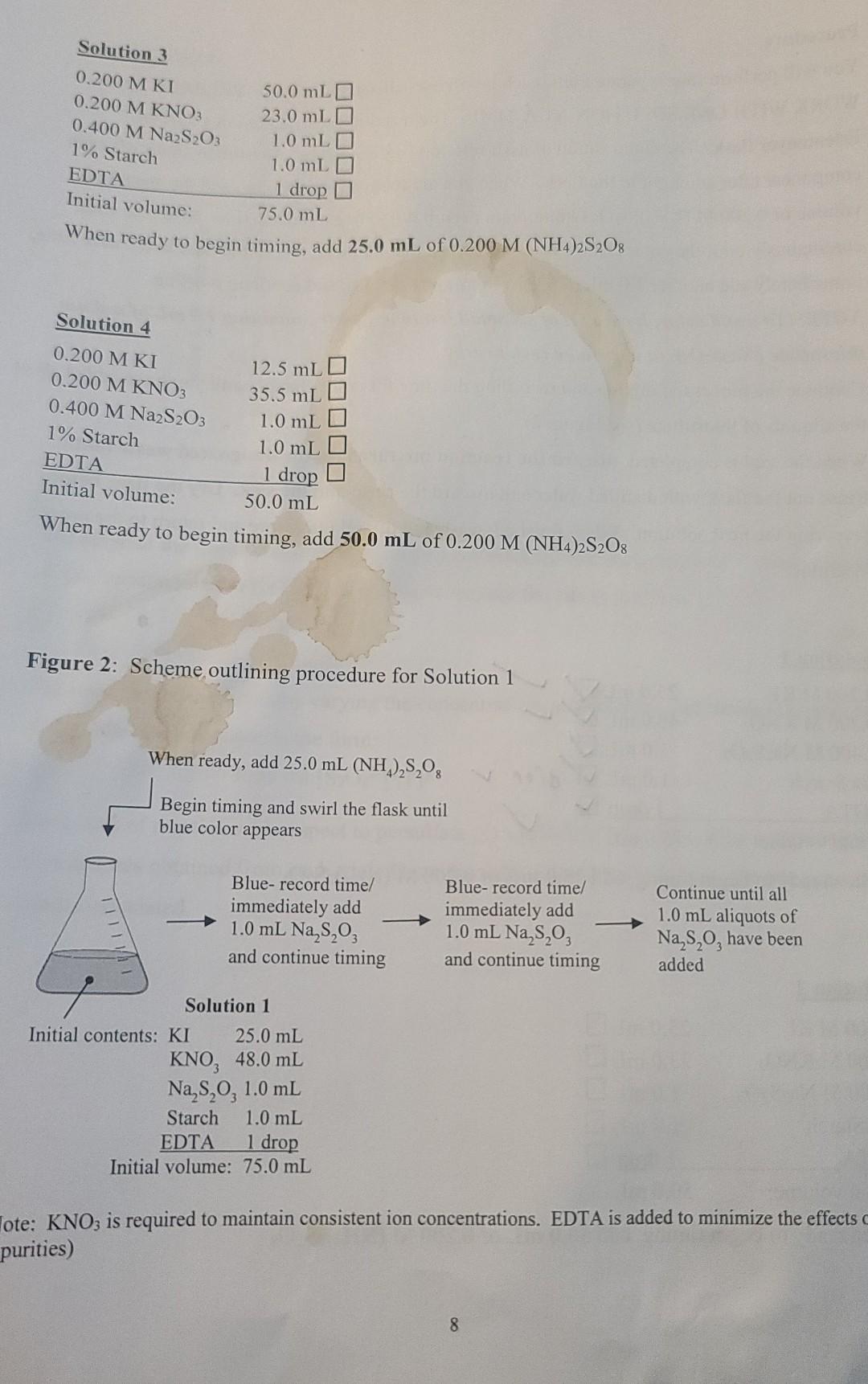

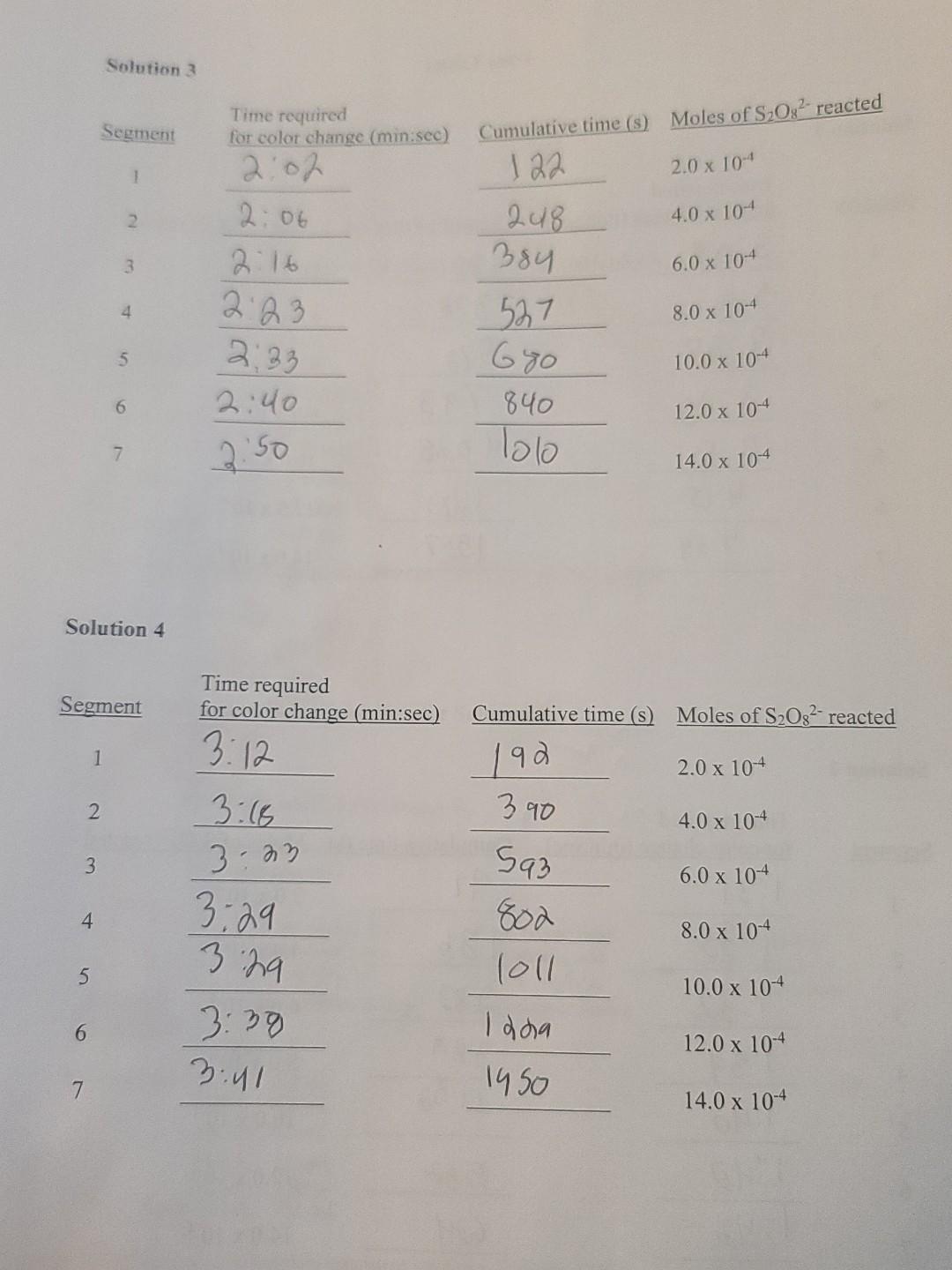


Procedure You will perform four separate trials, each involving a different solution. PREPARE AND WORK WITH ONE SOLUTION AT A TIME. The reaction is carried out in a 250 mL Erlenmeyer flask. The composition of each solution is given below. Be sure to check off each component after adding it to the flask. When you are ready to start timing, add the specified volume of 0.200 M (NH4)2S2O: (ammonium persulfate) to begin the reaction. Swirl the flask continuously until the color change is observed. At that moment, record the time in the data table, immediately add another 1.0 mL (4.0 x 10+ mol) of Na2S2O3, and continue swirling. NOTE: To avoid delay, have a set of six small test tubes, each containing 1.0 mL of sodium thiosulfate (Na2S2O3), in a test tube rack nearby. Continue the process of mixing and recording the time for color change until you have added all of the aliquots of thiosulfate (see Figure 2). When the trial is completed, discard the reaction mixture into the designated waste bottle. Rinse out the flask with distilled water and discard the rinse into the sink. Dry the flask before preparing the next solution. When finished, wash and dry all glassware and return to the proper locations. Solution 1 Is 0.200 MKI 0.200 M KNO3 0.400 M Na2S2O3 1% Starch EDTA Initial volume: 25.0 mL 48.0 mL 1.0 mL 1.0 mL drop v 1 drop V 75.0 mL When ready to begin timing, add 25.0 mL of 0.200 M (NH4)2S208 Solution 2 0.200 MKI 0.200 M KNO3 0.400 M Na2S2O3 1% Starch EDTA Initial volume: 25.0 mL O 23.0 mL O 1.0 mL O 1.0 mL O 1 drop 50.0 mL When ready to begin timing, add 50.0 mL of 0.200 M (NH4)2S208 Solution 3 0.200 MKI 0.200 M KNO3 0.400 M Na2S2O3 1% Starch EDTA Initial volume: 50.0 mL 23.0 mL 1.0 mLD 1.0 mL O 1 drop D 75.0 mL When ready to begin timing, add 25.0 mL of 0.200 M (NH4)2S2O: Solution 4 0.200 M KI 0.200 M KNO3 0.400 M Na2S2O3 1% Starch EDTA Initial volume: 12.5 mL 35.5 mL 1.0 mL 1.0 mL O 1 drop 0 50.0 mL When ready to begin timing, add 50.0 mL of 0.200 M (NH4)2S2O3 Figure 2: Scheme outlining procedure for Solution 1 When ready, add 25.0 mL (NH2),8,0 Begin timing and swirl the flask until blue color appears Blue- record time/ immediately add 1.0 mL Na 8,03 and continue timing Blue-record time! immediately add 1.0 mL Na,s,o, and continue timing Continue until all 1.0 mL aliquots of Na,,, have been added Solution 1 nitial contents: KI 25.0 mL KNO, 48.0 mL Na,s,o, 1.0 mL Starch 1.0 mL EDTA 1 drop Initial volume: 75.0 mL Tote: KNO3 is required to maintain consistent ion concentrations. EDTA is added to minimize the effects purities) 8 Data Tables Solution 1 Segment 1 Time required for color change (min:sec) 3:08 3:10 3:20 Cumulative time (s) Moles of S2082- reacted 188 2.0 x 104 328 4.0 x 104 2 578 6.0 x 10-4 3 793 8.0 x 10-4 4 3.35 3:55 10.0 x 104 5 4.15 1028 1 283 1557 12.0 x 10-4 6 4:34 14.0 x 10-4 7 Solution 2 Segment Cumulative time (s) Time required for color change (min:sec) 131 Moles of S2082 reacted 1 2.0 x 10-4 1:35 91 186 283 2 4.0 x 104 3 6.0 x 104 4 1:37 ):39 1" 380 1182 8.0 x 104 5 10.0 x 104 6 582 634 12.0 x 10-4 T: 42 7 14.0 x 10-4 9 Solution 3 Segment Time required for color change (min sec) 2:02 Cumulative time (s) Moles of S2082 reacted 122. 2.0 x 10-4 1 2 4.0 x 104 3 6.0 x 10-4 4. 2:06 2:16 2:23 2:33 2:40 8.0 x 10-4 248 384 527 680 840 lolo 5 10.0 x 10-4 6 12.0 x 10-4 7 2.50 14.0 x 10-4 Solution 4 Segment Time required for color change (min:sec) 3:12 Cumulative time (s) Moles of S2O32- reacted 192 2.0 x 10-4 1 2 390 4.0 x 10-4 3:16 3:23 3 6.0 x 10-4 4 3.29 3:29 593 802 loll 8.0 x 10-4 5 10.0 x 10-4 6 3:38 3:41 Idha 1950 12.0 x 10-4 7 14.0 x 10-4 For each solution you will have seven data points to graph. Based on the stoichiometry, 2.0 x 10-mol of persulfate has reacted at each interval. Using graph paper or a graphing program such as Excel, plot the moles of (NH4)2S2O: reacted versus cumulative time in seconds for each solution. Find the slope of the line for each trial. Remember that the total volume of each solution is 100.0 mL. Dividing the slope by the total volume of solution in liters (0.1000 L) gives the rate in M/s. Determine the order of reaction with respect to both persulfate and iodide ions, and write the rate law based on your results. e Calculate the rate constant (k) for each trial and then take the average. 1. Enter the rate of reaction determined for each solution. Solution 1: M/s Solution 2: M/s Solution 3: M/s Solution 4: M/s 2. Compare the reaction rates between solutions 1 and 2. Note that persulfate concentration was doubled in solution 2, while iodide concentration was held constant. 3. Compare the reaction rates between solutions 1 and 3. Note that iodide concentration was doubled in solution 3, while persulfate concentration was held constant. 4. Compare the reaction rates between solutions 2 and 4. Note that iodide concentration was cut in half in solution 4, while persulfate concentration was held constant. 5. Write the experimental rate law with respect to persulfate and iodide
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


