Question: In a batch distillation process, the still is initially charged with 105 kmols of a binary feed liquor in which the mole fraction of the
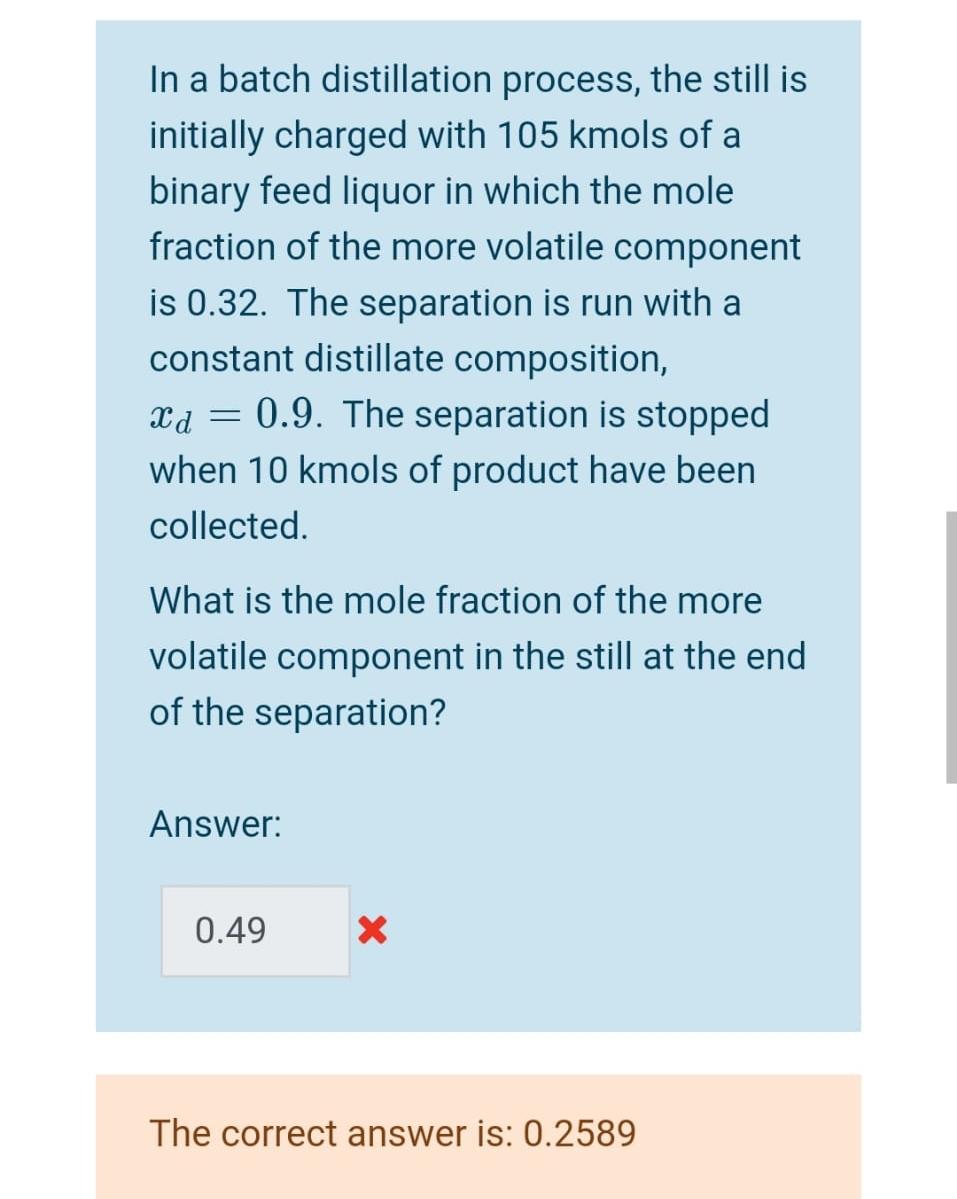

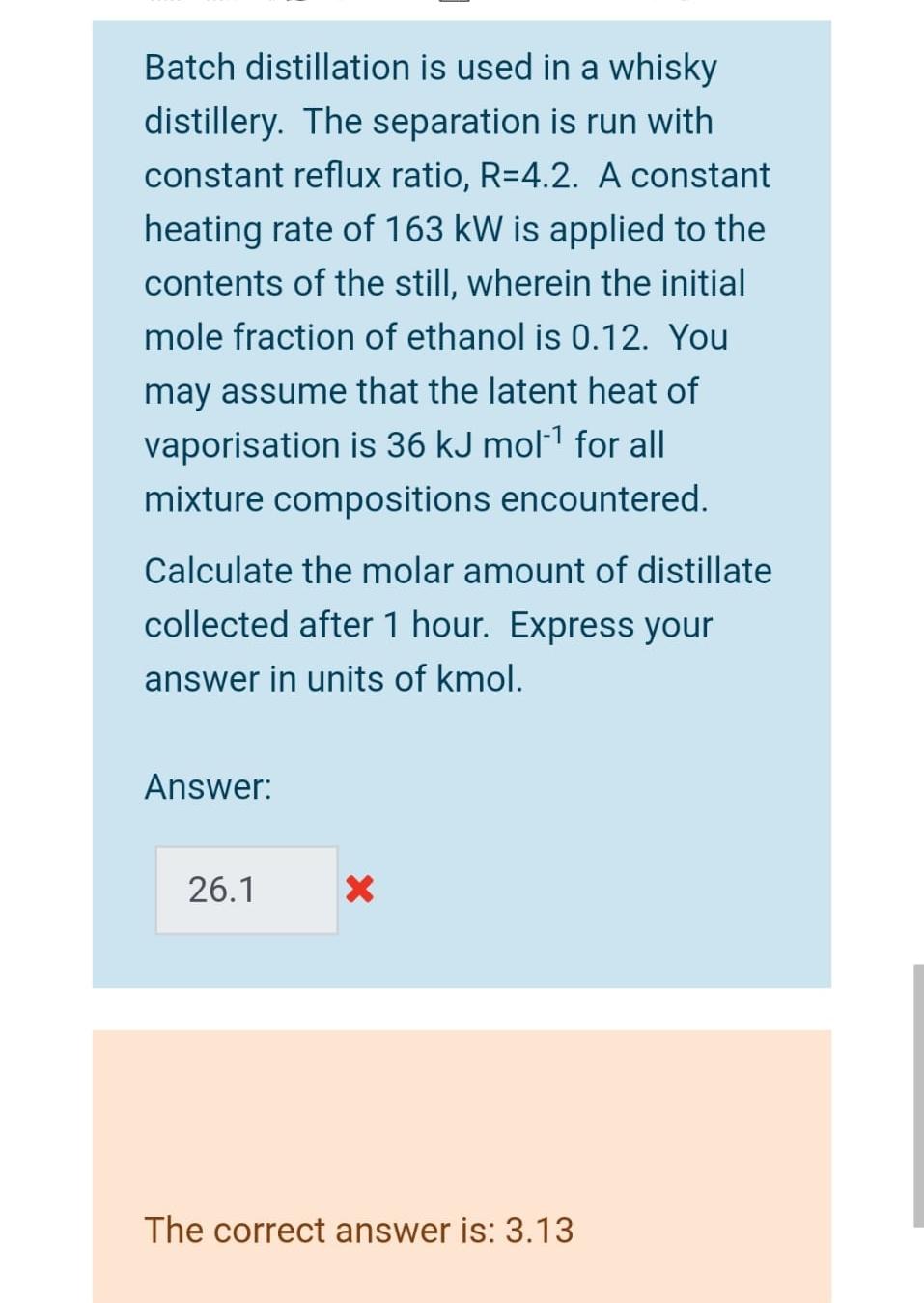
In a batch distillation process, the still is initially charged with 105 kmols of a binary feed liquor in which the mole fraction of the more volatile component is 0.32. The separation is run with a constant distillate composition, xd = 0.9. The separation is stopped when 10 kmols of product have been collected - What is the mole fraction of the more volatile component in the still at the end of the separation? Answer: 0.49 X The correct answer is: 0.2589 A continuous multi-stage distillation column processes a binary mixture. The mole fraction of the more volatile component in the distillate is 0.9. The reflux ratio is 0.3. For the section of the column above the feed, the equilibrium line can be approximated by a straight line with gradient, 2.5. What is the mole fraction of the more volatile component in the vapour leaving the second-top stage of the column, i.e. Yn-1. Answer: 0.29 X The correct answer is: 0.733 Batch distillation is used in a whisky distillery. The separation is run with constant reflux ratio, R=4.2. A constant heating rate of 163 kW is applied to the contents of the still, wherein the initial mole fraction of ethanol is 0.12. You may assume that the latent heat of vaporisation is 36 kJ mol-1 for all mixture compositions encountered. Calculate the molar amount of distillate collected after 1 hour. Express your answer in units of kmol. Answer: 26.1 X The correct answer is: 3.13
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


