Question: A batch distillation process is used to reclaim a mixture of water (HO) and hydrogen peroxide (HO) used for the wet etching of tungsten
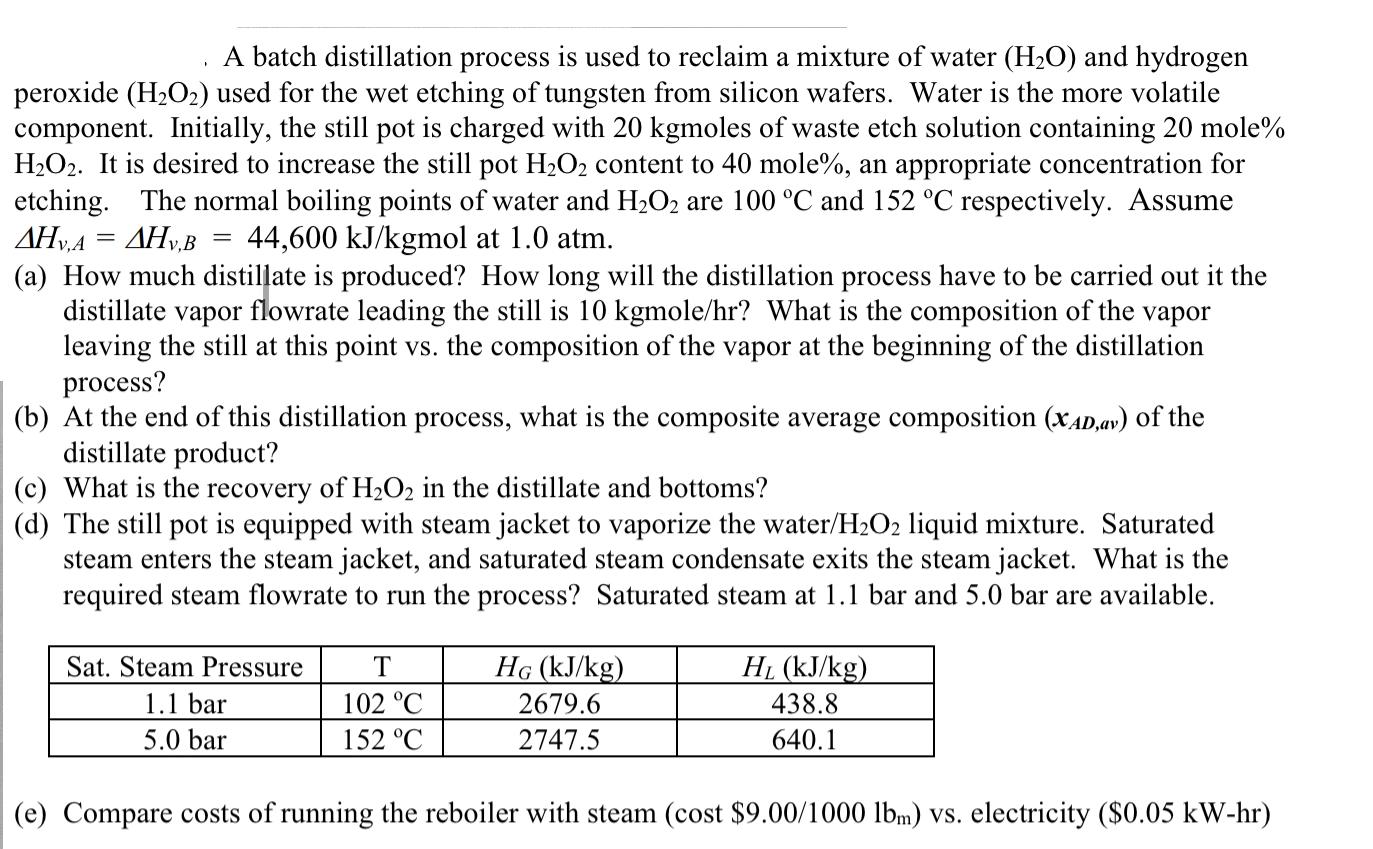
A batch distillation process is used to reclaim a mixture of water (HO) and hydrogen peroxide (HO) used for the wet etching of tungsten from silicon wafers. Water is the more volatile component. Initially, the still pot is charged with 20 kgmoles of waste etch solution containing 20 mole% HO2. It is desired to increase the still pot HO content to 40 mole%, an appropriate concentration for etching. The normal boiling points of water and HO2 are 100 C and 152 C respectively. Assume AHVA = AHV,B 44,600 kJ/kgmol at 1.0 atm. (a) How much distillate is produced? How long will the distillation process have to be carried out it the distillate vapor flowrate leading the still is 10 kgmole/hr? What is the composition of the vapor leaving the still at this point vs. the composition of the vapor at the beginning of the distillation process? (b) At the end of this distillation process, what is the composite average composition (XAD,av) of the distillate product? (c) What is the recovery of HO2 in the distillate and bottoms? (d) The still pot is equipped with steam jacket to vaporize the water/HO2 liquid mixture. Saturated steam enters the steam jacket, and saturated steam condensate exits the steam jacket. What is the required steam flowrate to run the process? Saturated steam at 1.1 bar and 5.0 bar are available. Sat. Steam Pressure 1.1 bar 5.0 bar T 102 C 152 C HG (kJ/kg) 2679.6 2747.5 HL (kJ/kg) 438.8 640.1 (e) Compare costs of running the reboiler with steam (cost $9.00/1000 lbm) vs. electricity ($0.05 kW-hr)
Step by Step Solution
3.37 Rating (156 Votes )
There are 3 Steps involved in it
a To determine the amount of distillate produced we need to calculate the minimum number of stages required for the distillation process The minimum number of stages can be determined using the Fenske ... View full answer

Get step-by-step solutions from verified subject matter experts


