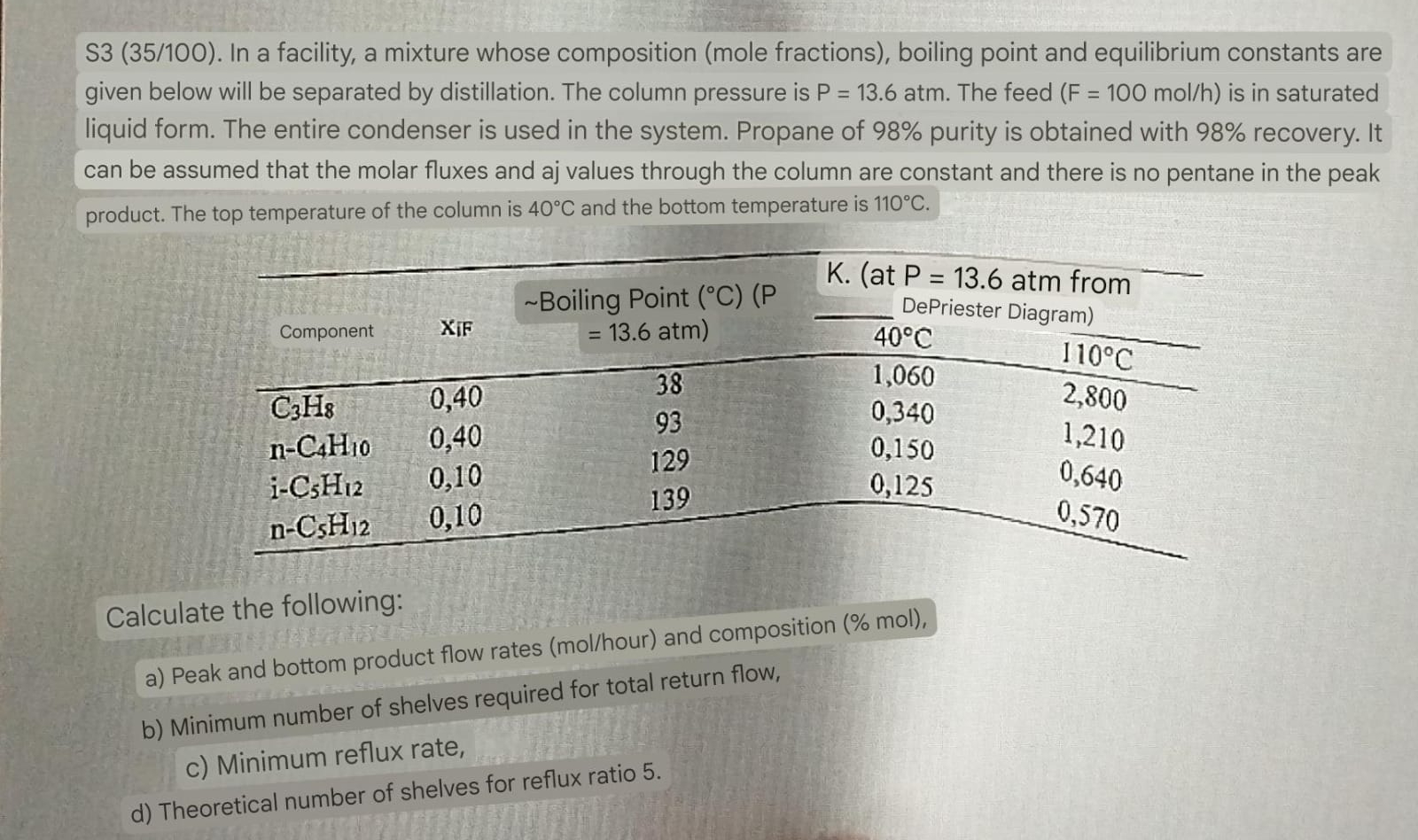
Question: In a facility, a mixture whose composition ( mole fractions ) , boiling point and equilibrium constants are given below will be separated by distillation.
In a facility, a mixture whose composition mole fractions boiling point and equilibrium constants are
given below will be separated by distillation. The column pressure is atm. The feed is in saturated
liquid form. The entire condenser is used in the system. Propane of purity is obtained with recovery. It
can be assumed that the molar fluxes and aj values through the column are constant and there is no pentane in the peak
product. The top temperature of the column is and the bottom temperature is
Calculate the following:
a Peak and bottom product flow rates molhour and composition mol
b Minimum number of shelves required for total return flow,
c Minimum reflux rate,
d Theoretical number of shelves for reflux ratio
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


