Question: In a gas phase plug flow reactor, the elementary reaction occurs: C6H6(g)+C2H3Cl(s)C7H8(G)]+CH4(g) What is the standard heat of formation of the reaction at 25C. What
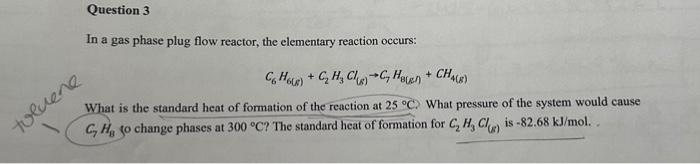
In a gas phase plug flow reactor, the elementary reaction occurs: C6H6(g)+C2H3Cl(s)C7H8(G)]+CH4(g) What is the standard heat of formation of the reaction at 25C. What pressure of the system would cause C7H8 to change phases at 300C ? The standard heat of formation for C2H3Cl(g) is 82.68kJ/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


