Question: Consider the reversible elementary gas phase reaction CO + H20 2 CO2 + H2 The reaction is carried out in a, adiabatic, plug flow reactors
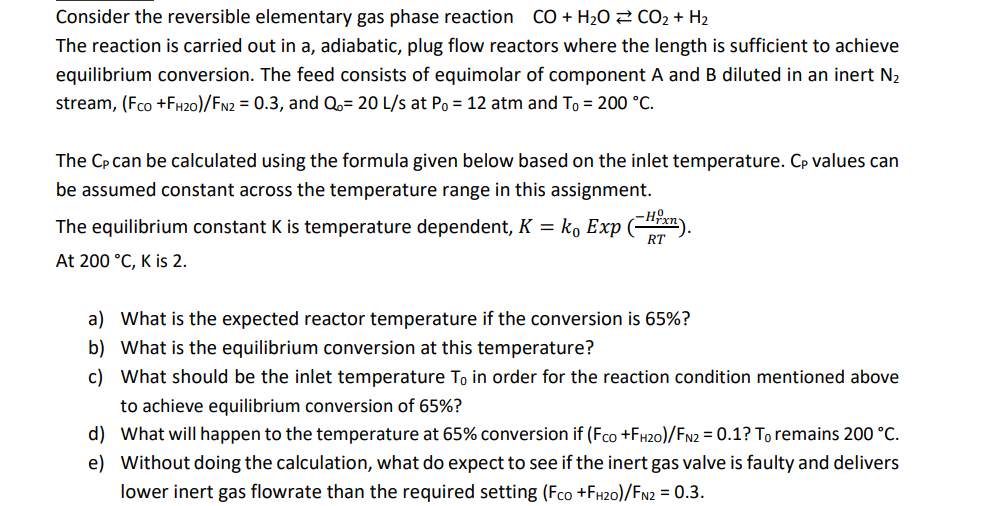
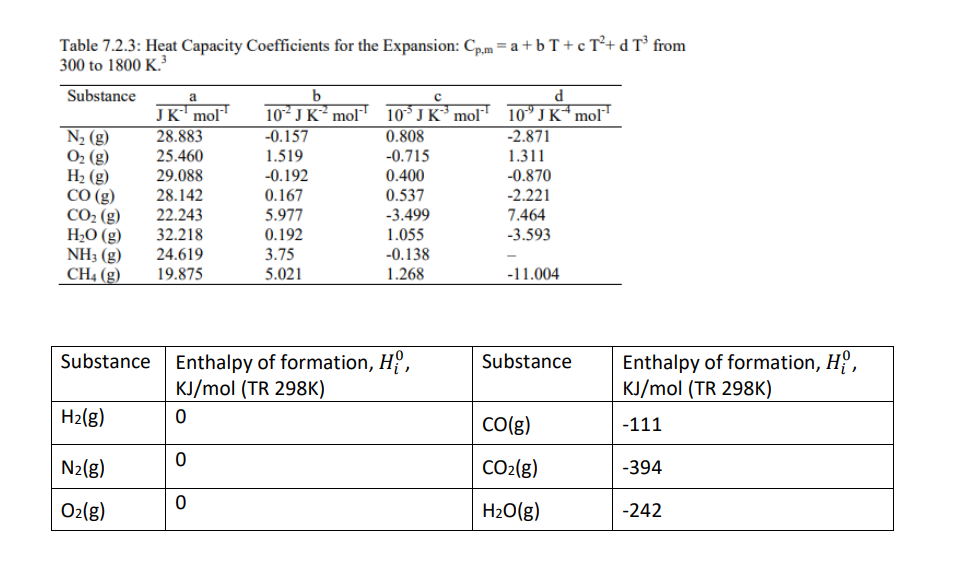
Consider the reversible elementary gas phase reaction CO + H20 2 CO2 + H2 The reaction is carried out in a, adiabatic, plug flow reactors where the length is sufficient to achieve equilibrium conversion. The feed consists of equimolar of component A and B diluted in an inert N2 stream, (Fco +FH20)/FN2 = 0.3, and Q.= 20 L/s at Po = 12 atm and To = 200 C. The Cp can be calculated using the formula given below based on the inlet temperature. Cp values can be assumed constant across the temperature range in this assignment. The equilibrium constant K is temperature dependent, K = ko Exp Chixn). At 200 C, K is 2. RT a) What is the expected reactor temperature if the conversion is 65%? b) What is the equilibrium conversion at this temperature? c) What should be the inlet temperature To in order for the reaction condition mentioned above to achieve equilibrium conversion of 65%? d) What will happen to the temperature at 65% conversion if (Fco +FH20)/FN2 = 0.1? To remains 200 C. e) Without doing the calculation, what do expect to see if the inert gas valve is faulty and delivers lower inert gas flowrate than the required setting (Fco +FH20)/FN2 = 0.3. Table 7.2.3: Heat Capacity Coefficients for the Expansion: Cp.m=a+bT+c T2+d T from 300 to 1800 K.? Substance 10* J K mol N2 (g) O2 (g) H2 (g) CO(g) CO2 (g) H2O(g) NH; (g) CH4 (g) a JK mol' 28.883 25.460 29.088 28.142 22.243 32.218 24.619 19.875 b 10- JK mol -0.157 1.519 -0.192 0.167 5.977 0.192 3.75 5.021 0.808 -0.715 0.400 0.537 -3.499 1.055 -0.138 1.268 d 10'J K mol' -2.871 1.311 -0.870 -2.221 7.464 -3.593 -11.004 Substance Substance Enthalpy of formation, H, KJ/mol (TR 298K) H2(g) 0 Enthalpy of formation, H, KJ/mol (TR 298K) CO(g) -111 0 N2(g) CO2(g) -394 0 O2(g) H2O(g) -242
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


