Question: . In a process, an acid ( 1 ) is mixed with water ( 2 ) to form an equimolar mixture in a open vessel
In a process, an acid is mixed with water to form an equimolar mixture in a open vessel constant P The
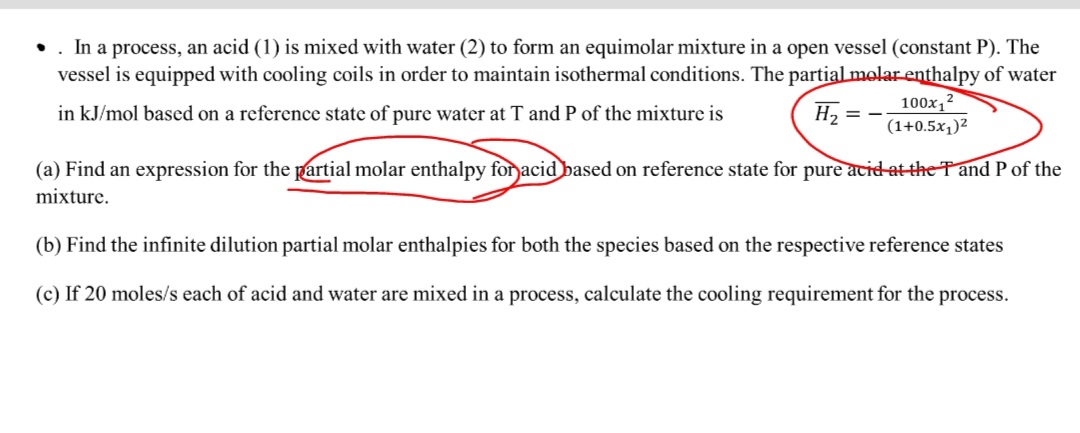
vessel is equipped with cooling coils in order to maintain isothermal conditions. The nartial molar enthalnv of water
in based on a reference state of pure water at and of the mixture is
a Find an expression for the partial molar enthalpy for acidbased on reference state for pure acid at the T and of the
mixture.
b Find the infinite dilution partial molar enthalpies for both the species based on the respective reference states
c If mole each of acid and water are mixed in a process, calculate the cooling requirement for the process.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


