Question: In a process, solid MX2 is reacted with water vapor-Y2 gas mixture (Y2 inert gas) to produce solid metal oxide (MO). A mixture of
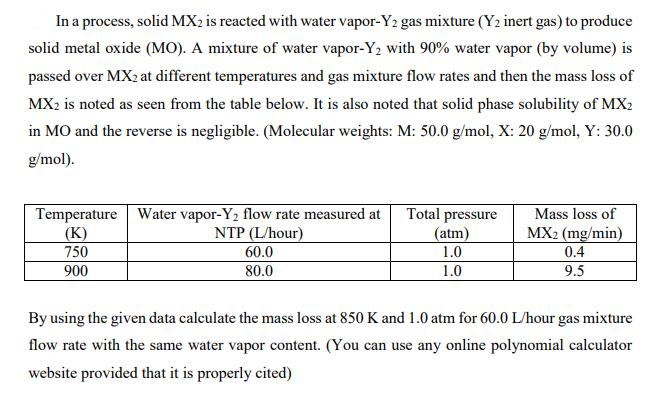
In a process, solid MX2 is reacted with water vapor-Y2 gas mixture (Y2 inert gas) to produce solid metal oxide (MO). A mixture of water vapor-Y2 with 90% water vapor (by volume) is passed over MX2 at different temperatures and gas mixture flow rates and then the mass loss of MX2 is noted as seen from the table below. It is also noted that solid phase solubility of MX2 in MO and the reverse is negligible. (Molecular weights: M: 50.0 g/mol, X: 20 g/mol, Y: 30.0 g/mol). Temperature Water vapor-Y2 flow rate measured at (K) 750 900 Total pressure (atm) Mass loss of MX2 (mg/min) 0.4 9.5 NTP (L/hour) 60.0 1.0 80.0 1.0 By using the given data calculate the mass loss at 850 K and 1.0 atm for 60.0 L/hour gas mixture flow rate with the same water vapor content. (You can use any online polynomial calculator website provided that it is properly cited)
Step by Step Solution
3.44 Rating (167 Votes )
There are 3 Steps involved in it
t2 for of H2o TC MAR MYE is loss Ma... View full answer

Get step-by-step solutions from verified subject matter experts


