Question: In aqueous solution, sucrose, 3, can react to form glucose and fructose. This process is acid catalysed, beginning with ether oxygen protonation. Table 2 gives
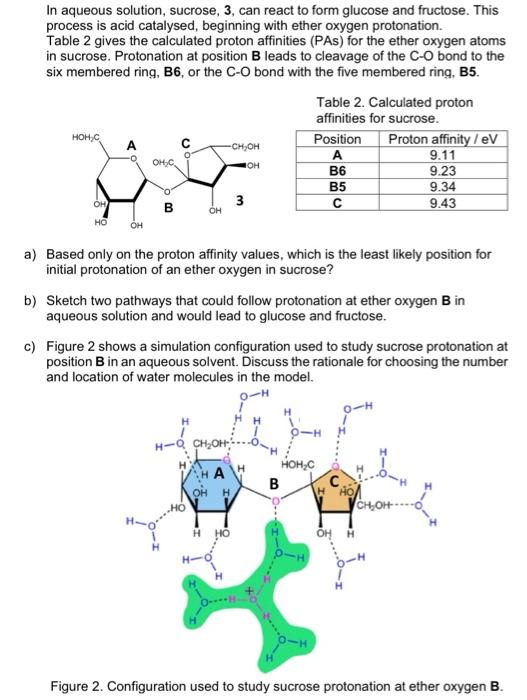
In aqueous solution, sucrose, 3, can react to form glucose and fructose. This process is acid catalysed, beginning with ether oxygen protonation. Table 2 gives the calculated proton affinities (PAs) for the ether oxygen atoms in sucrose. Protonation at position B leads to cleavage of the C-O bond to the six membered ring, B6, or the C-O bond with the five membered ring, B5. Table 2. Calculated proton affinities for sucrose. Position Proton affinity / eV A 9.11 9.23 9.34 3 C 9.43 HOHC C CH, OH OH B6 B5 OH B OH HO OH a) Based only on the proton affinity values, which is the least likely position for initial protonation of an ether oxygen in sucrose? b) Sketch two pathways that could follow protonation at ether oxygen B in aqueous solution and would lead to glucose and fructose. c) Figure 2 shows a simulation configuration used to study sucrose protonation at position B in an aqueous solvent. Discuss the rationale for choosing the number and location of water molecules in the model. O-H H HH O-H H-OCH OH VAHA HO B WHO CH, OH ---- Figure 2. Configuration used to study sucrose protonation at ether oxygen B. In aqueous solution, sucrose, 3, can react to form glucose and fructose. This process is acid catalysed, beginning with ether oxygen protonation. Table 2 gives the calculated proton affinities (PAs) for the ether oxygen atoms in sucrose. Protonation at position B leads to cleavage of the C-O bond to the six membered ring, B6, or the C-O bond with the five membered ring, B5. Table 2. Calculated proton affinities for sucrose. Position Proton affinity / eV A 9.11 9.23 9.34 3 C 9.43 HOHC C CH, OH OH B6 B5 OH B OH HO OH a) Based only on the proton affinity values, which is the least likely position for initial protonation of an ether oxygen in sucrose? b) Sketch two pathways that could follow protonation at ether oxygen B in aqueous solution and would lead to glucose and fructose. c) Figure 2 shows a simulation configuration used to study sucrose protonation at position B in an aqueous solvent. Discuss the rationale for choosing the number and location of water molecules in the model. O-H H HH O-H H-OCH OH VAHA HO B WHO CH, OH ---- Figure 2. Configuration used to study sucrose protonation at ether oxygen B
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


