Question: In Chapter 20, we will explore how nitriles can be converted into carboxylic acids. How would you use IR spectroscopy to monitor the progress of
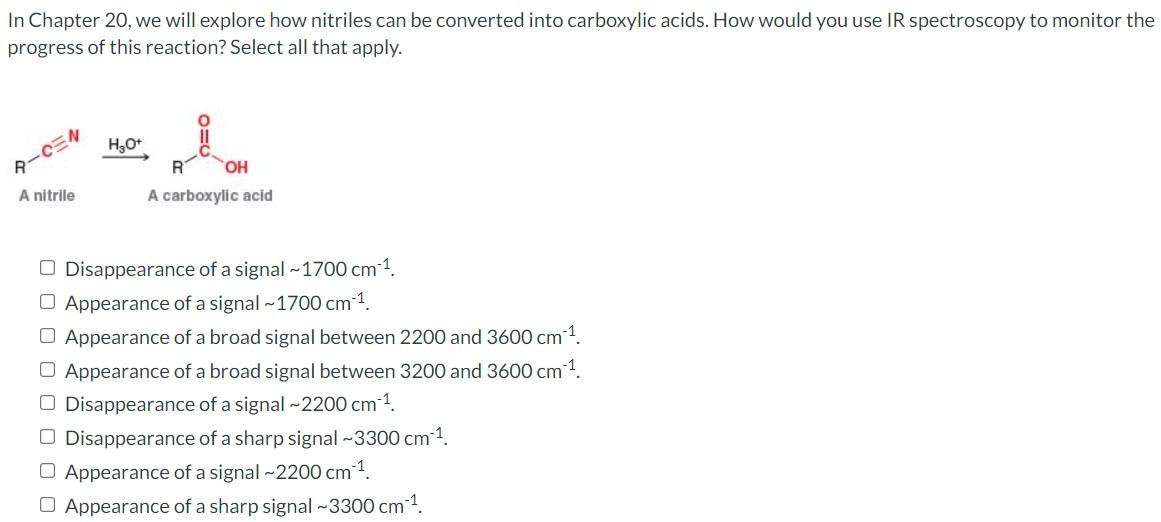
In Chapter 20, we will explore how nitriles can be converted into carboxylic acids. How would you use IR spectroscopy to monitor the progress of this reaction? Select all that apply. A-OEN H20+ R OH A carboxylic acid A nitrile Disappearance of a signal - 1700 cm 1. O Appearance of a signal - 1700 cm-1 Appearance of a broad signal between 2200 and 3600 cm-1 O Appearance of a broad signal between 3200 and 3600 cm 1 -1 O Disappearance of a signal -2200 cm-1 Disappearance of a sharp signal -3300 cm 1 Appearance of a signal ~2200 cm 1. Appearance of a sharp signal -3300 cm-1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


