Question: In - Class Example 2 6 : Ethane ( at 2 0 0 kPa and ( 5 0 ^ { circ }
InClass Example :
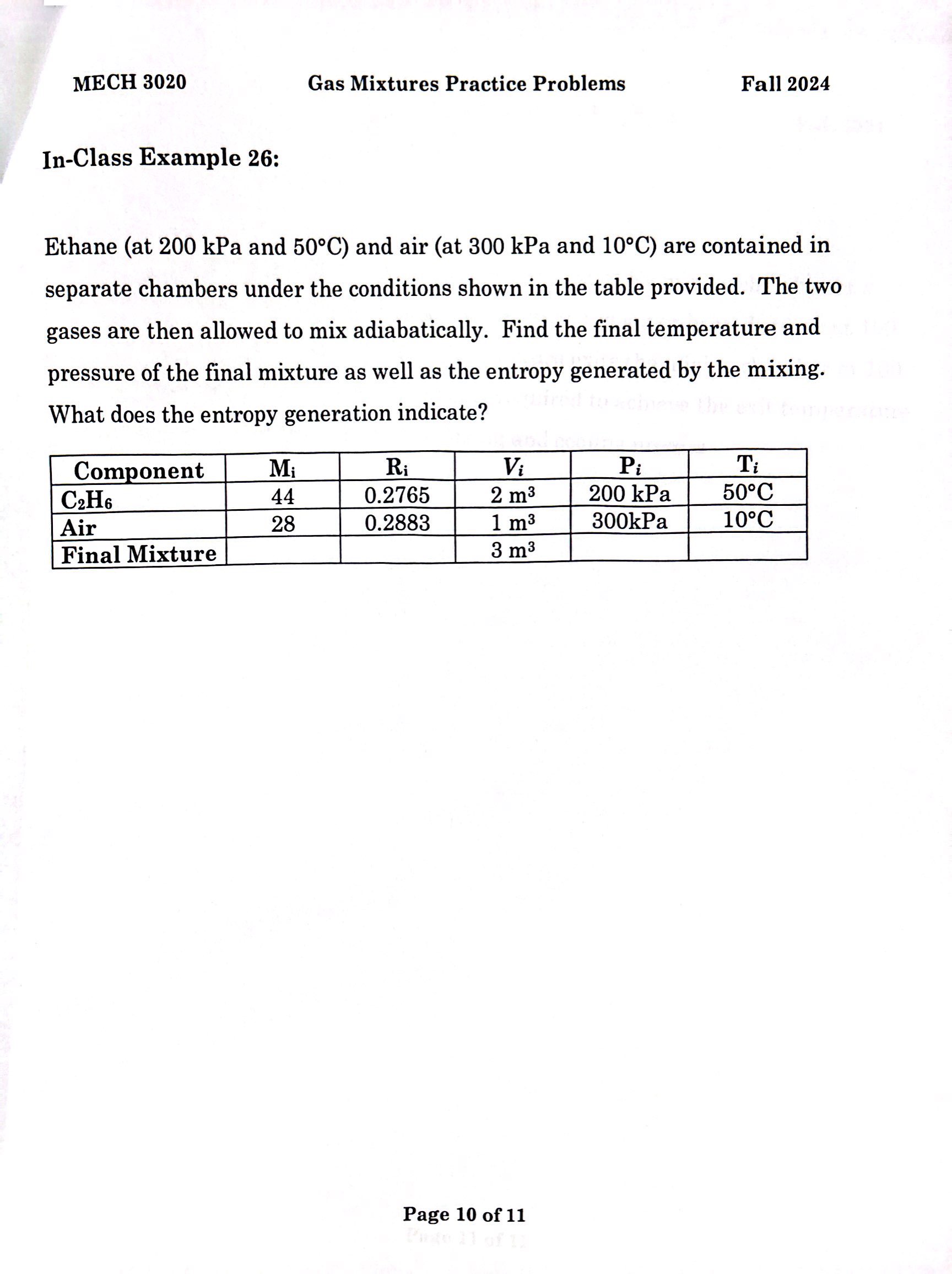
Ethane at kPa and circmathrmC and air at kPa and circmathrmC are contained in separate chambers under the conditions shown in the table provided. The two gases are then allowed to mix adiabatically. Find the final temperature and pressure of the final mixture as well as the entropy generated by the mixing. What does the entropy generation indicate?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


