Question: in KINETIC STUDY USING SPECTROSCOPY lab experiment. we used two beakers for our sample as shown in order to calculate the reaction rate and the
in KINETIC STUDY USING SPECTROSCOPY lab experiment. we used two beakers for our sample as shown in order to calculate the reaction rate and the order of the reaction, the question is why did we use NaCl?
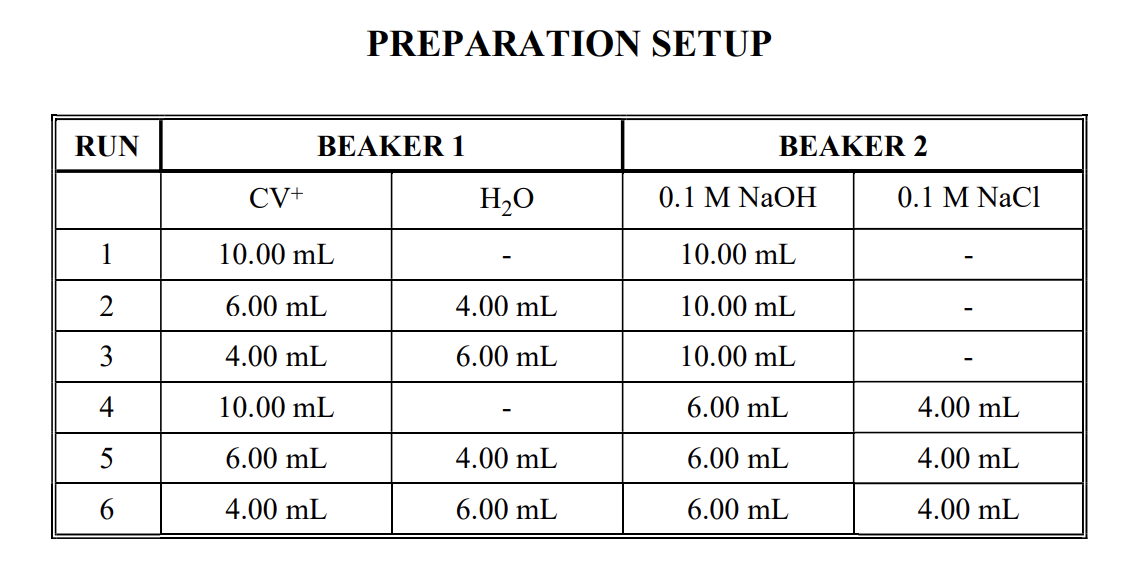
PREPARATION SETUP RUN BEAKER 1 BEAKER 2 CV+ HO 0.1 M NaOH 0.1 M NaCl 1 10.00 mL 10.00 mL 2 6.00 mL 4.00 mL 10.00 mL 3. 4.00 mL 6.00 mL 10.00 mL 4 10.00 mL 6.00 mL 4.00 mL 5 6.00 mL 4.00 mL 6.00 mL 4.00 mL 6 4.00 mL 6.00 mL 6.00 mL 4.00 mL
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


