Question: A) A sample of an ideal gas undergo an isobaric process. Given that the pressure is 1 atm, and the volume of ideal gas
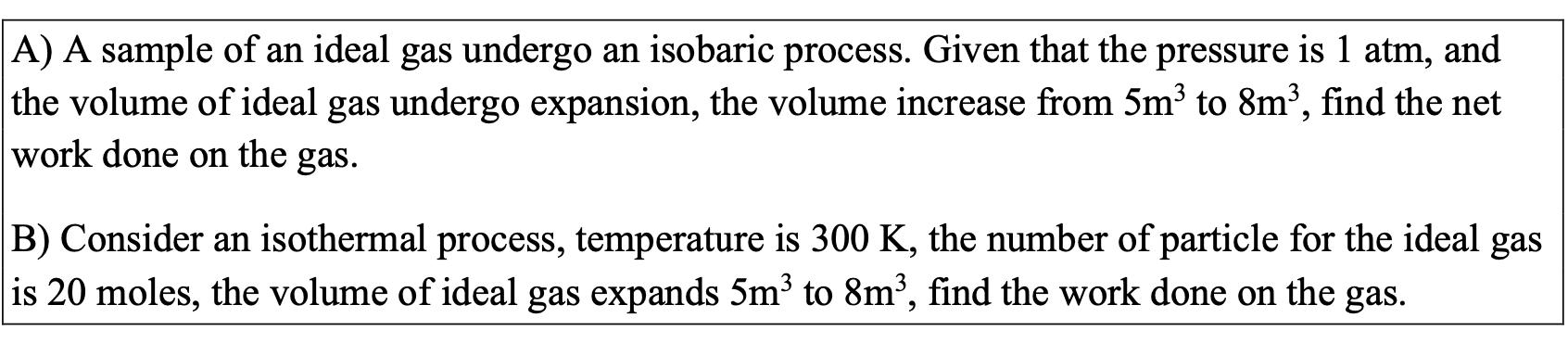
A) A sample of an ideal gas undergo an isobaric process. Given that the pressure is 1 atm, and the volume of ideal gas undergo expansion, the volume increase from 5m to 8m, find the net work done on the gas. B) Consider an isothermal process, temperature is 300 K, the number of particle for the ideal gas is 20 moles, the volume of ideal gas expands 5m to 8m, find the work done on the gas.
Step by Step Solution
3.46 Rating (156 Votes )
There are 3 Steps involved in it
A In an isobaric process the pressure remains constant The work done on or by the gas can be calcula... View full answer

Get step-by-step solutions from verified subject matter experts


