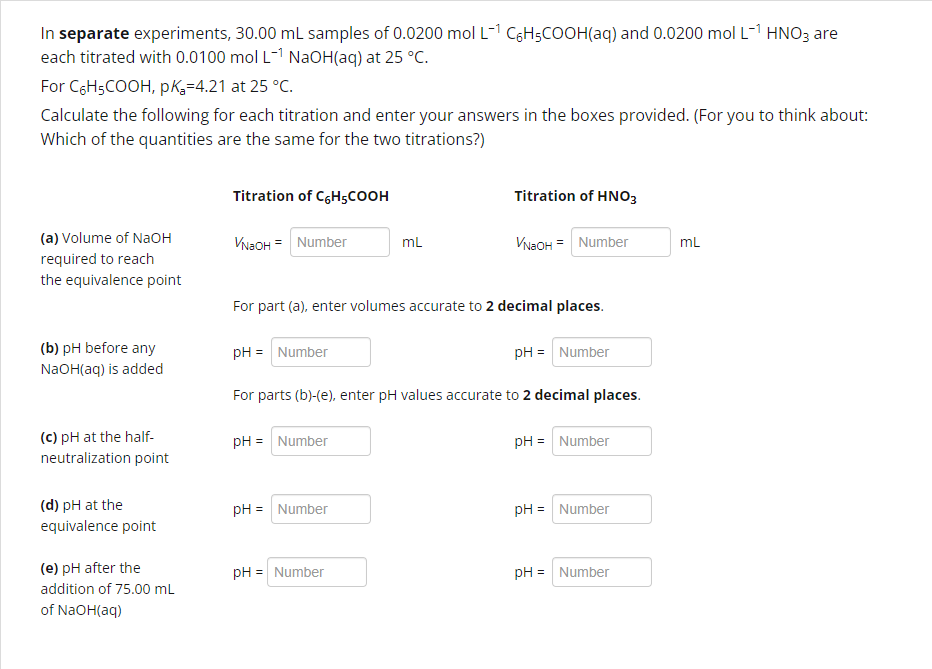
Question: In separate experiments, 3 0 . 0 0 mL samples of 0 . 0 2 0 0 mol L 1 C 6 H 5 COOH
In separate experiments, mL samples of mol L CHCOOHaq and mol L HNO are each titrated with mol L NaOHaq at deg C
For CHCOOH, pKa at deg C
Calculate the following for each titration and enter your answers in the boxes provided. For you to think about: Which of the quantities are the same for the two titrations?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


