Question: In the class we talked about the velocity distribution function of ideal monatomic gas f(v) = 4 pi N (m/2 pi k_B T)^3/2 v^2 exp
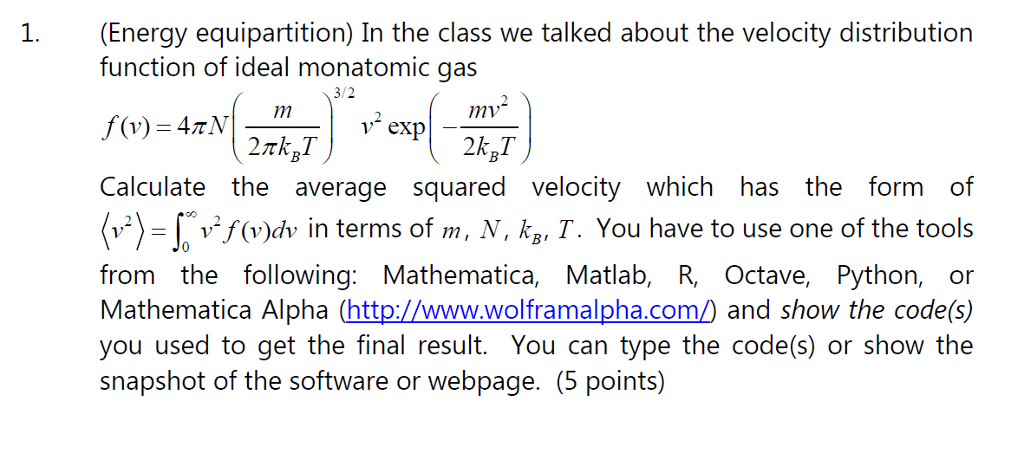
In the class we talked about the velocity distribution function of ideal monatomic gas f(v) = 4 pi N (m/2 pi k_B T)^3/2 v^2 exp (- mv^2/2 k_B T) Calculate the average squared velocity which has the form of (v^2) = integral^infinity_0 f (v) dv in terms of m, N, k_B, T. You have to use one of the tools from the following: Mathematica, Matlab, R, Octave, Python, or Mathematica Alpha (http://www.wolframalpha.com/) and show the code(s) you used to get the final result. You can type the code(s) or show the snapshot of the software or webpage
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


