Question: In the equation seen below, the reactant that is oxidized is: 2I(aq)+F2(g)>2F(aq)+I2(s) Select one: a. F(aq) b. I2(s) c. F(aq) d. F2(g)
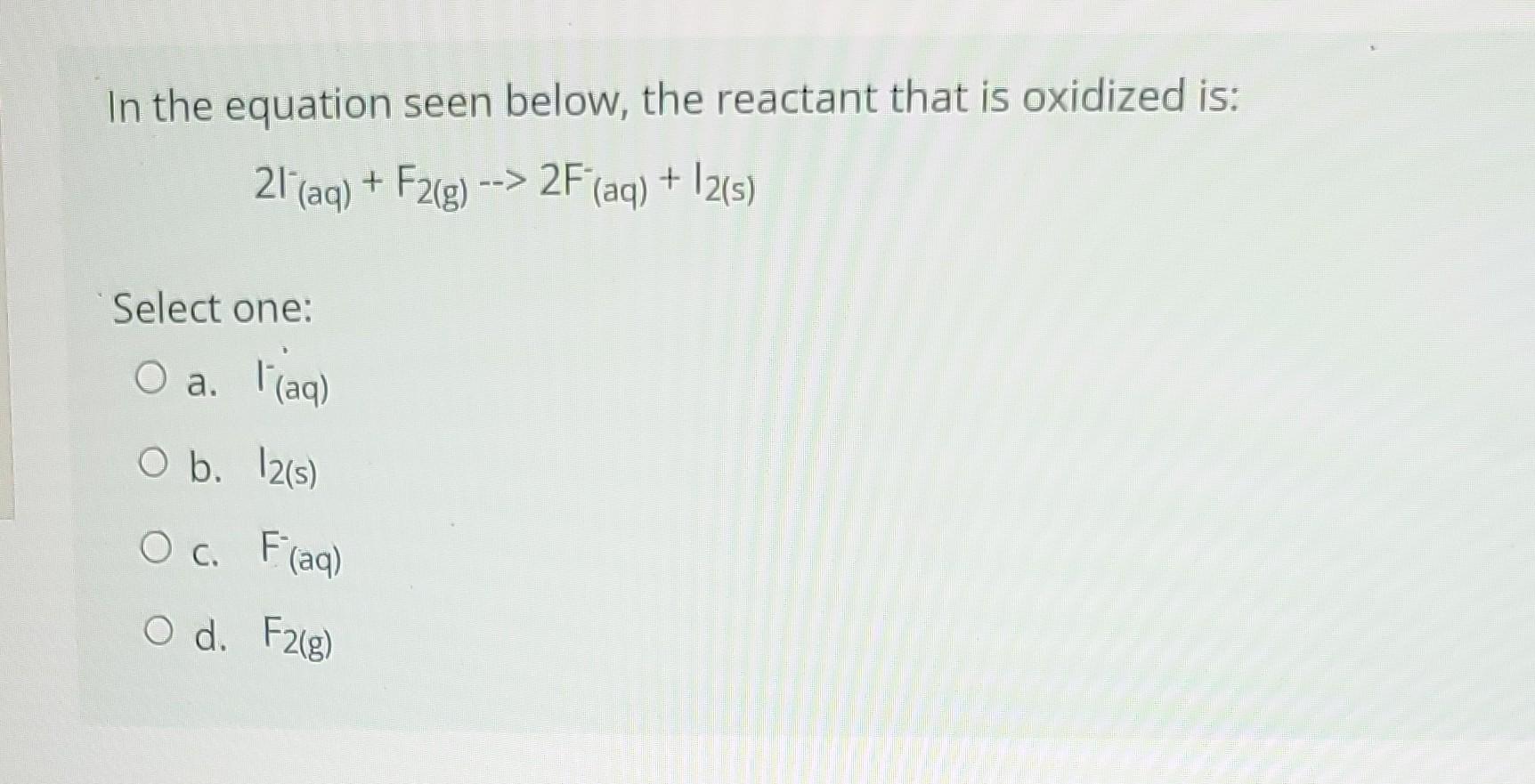
In the equation seen below, the reactant that is oxidized is: 2I(aq)+F2(g)>2F(aq)+I2(s) Select one: a. F(aq) b. I2(s) c. F(aq) d. F2(g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


