Question: In the famous Haber process ( Figure E 5 . 1 0 ) used to manufacture ammonia, the reaction is carried out at pressures of
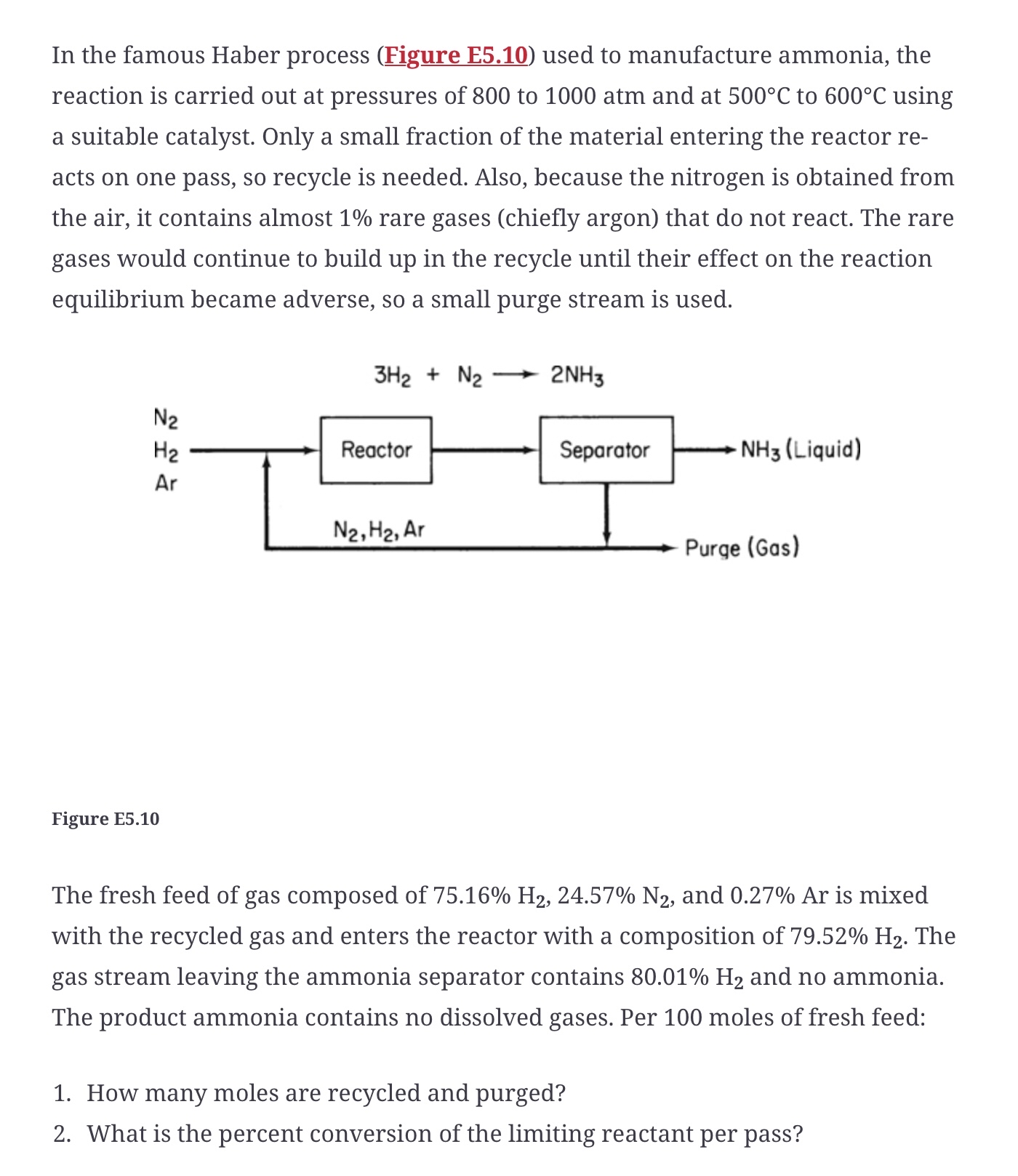
In the famous Haber process Figure E used to manufacture ammonia, the reaction is carried out at pressures of to atm and at to using a suitable catalyst. Only a small fraction of the material entering the reactor reacts on one pass, so recycle is needed. Also, because the nitrogen is obtained from the air, it contains almost rare gases chiefly argon that do not react. The rare gases would continue to build up in the recycle until their effect on the reaction equilibrium became adverse, so a small purge stream is used.
Figure E
The fresh feed of gas composed of and is mixed with the recycled gas and enters the reactor with a composition of The gas stream leaving the ammonia separator contains and no ammonia. The product ammonia contains no dissolved gases. Per moles of fresh feed:
How many moles are recycled and purged?
What is the percent conversion of the limiting reactant per pass?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


