Question: In the following problem, why do you not convert m^3/mol to m^3/kmol. The solution did not do this but it was correct in doing so.
In the following problem, why do you not convert m^3/mol to m^3/kmol. The solution did not do this but it was correct in doing so.
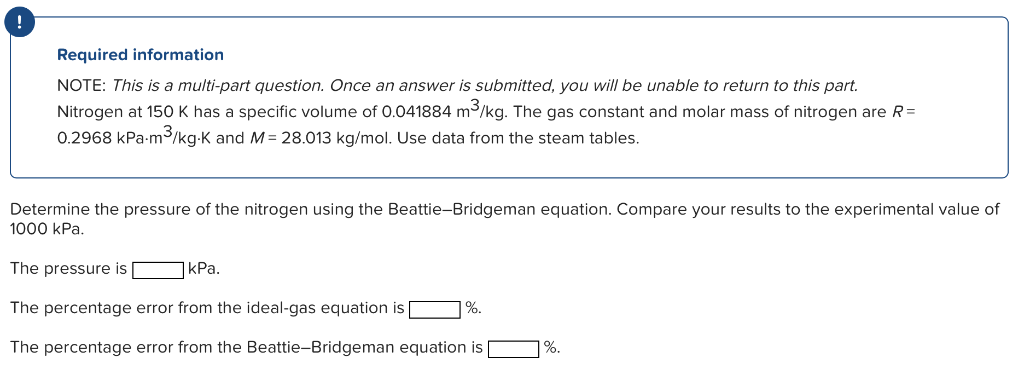
Required information NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part. Nitrogen at 150K has a specific volume of 0.041884m3/kg. The gas constant and molar mass of nitrogen are R= 0.2968kPam3/kgK and M=28.013kg/mol. Use data from the steam tables. Determine the pressure of the nitrogen using the Beattie-Bridgeman equation. Compare your results to the experimental value of 1000kPa The pressure is kPa. The percentage error from the ideal-gas equation is % The percentage error from the Beattie-Bridgeman equation is \%
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


