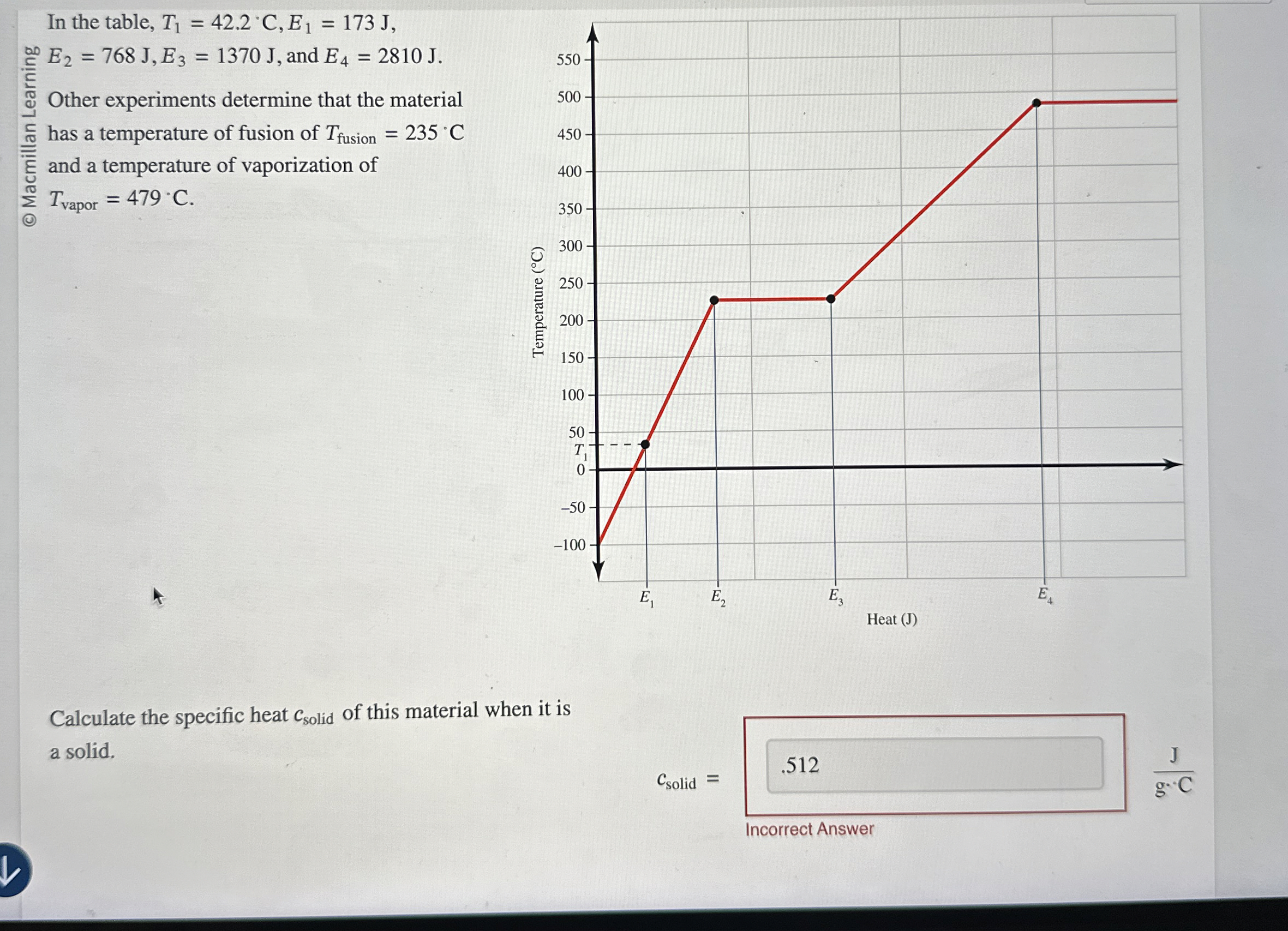
Question: In the table, T 1 = 4 2 . 2 C , E 1 = 1 7 3 J , E 2 = 7 6
In the table, and
Other experiments determine that the material has a temperature of fusion of and a temperature of vaporization of
Calculate the specific heat of this material when it is a solid.
Calculate the specific heat of this material when it is a liquid
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


