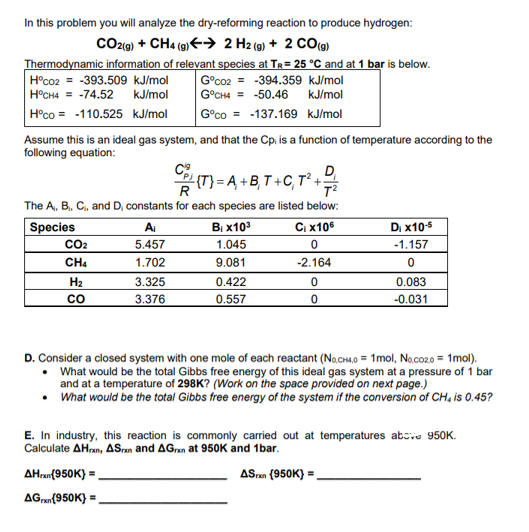
Question: In this problem you will analyze the dry - reforming reaction to produce hydrogen: C O 2 ( g ) + C H 4 (
In this problem you will analyze the dryreforming reaction to produce hydrogen:
harr
Thermodynamic information of relevant species at and at is below.
Assume this is an ideal gas system, and that the is a function of temperature according to the
following equation:
The and constants for each species are listed below:
D Consider a closed system with one mole of each reactant
What would be the total Gibbs free energy of this ideal gas system at a pressure of bar
and at a temperature of Work on the space provided on next page.
What would be the total Gibbs free energy of the system if the conversion of is
E In industry, this reaction is commonly carried out at temperatures atzo
Calculate and at and
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


