Question: In this problem you will consider the changes to the hydrogen 3d-states (principal quantum number n = 3 and orbital angular momentum l = 2)
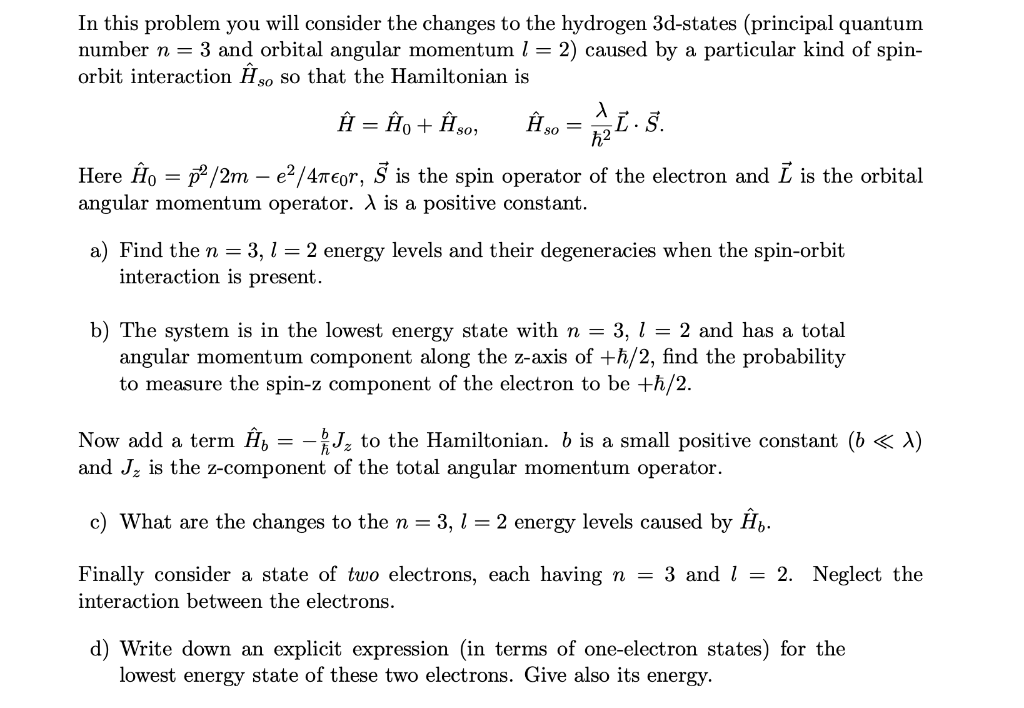
In this problem you will consider the changes to the hydrogen 3d-states (principal quantum number n = 3 and orbital angular momentum l = 2) caused by a particular kind of spin orbit interaction Hm so that the Hamiltonian is a .. A A A .. .. H2H0+H30, Hsosz-S. Here .FAIO = 359/2111. e2 ,1\" 4113501", 5\" is the spin operator of the electron and E is the orbital angular momentum operator. A is a positive constant. a) Find the n = 3, l = 2 energy levels and their degeneracies when the spin-orbit interaction is present. b) The system is in the lowest energy state with n = 3, l = 2 and has a total angular momentum component along the z-axis of +h/2, nd the probability to measure the spinz component of the electron to be +h/ 2. Now add a term H5 2 %Jz to the Hamiltonian. .5 is a small positive constant (b j: A) and Jz is the z-comp onent of the total angular momentum operator. 0) What are the changes to the to = 3, E = 2 energy levels caused by I35. Finally consider a state of two electrons, each having n. = 3 and E = 2. Neglect the interaction between the electrons. d) Write down an explicit expressiOn (in terms of One-electmn states) for the lowest energy state of these two electrons. Give also its energy
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


