Question: In year 2 2 4 3 , based on samples collected during missions to a moon of exoplanet Kepler 4 3 3 b , the
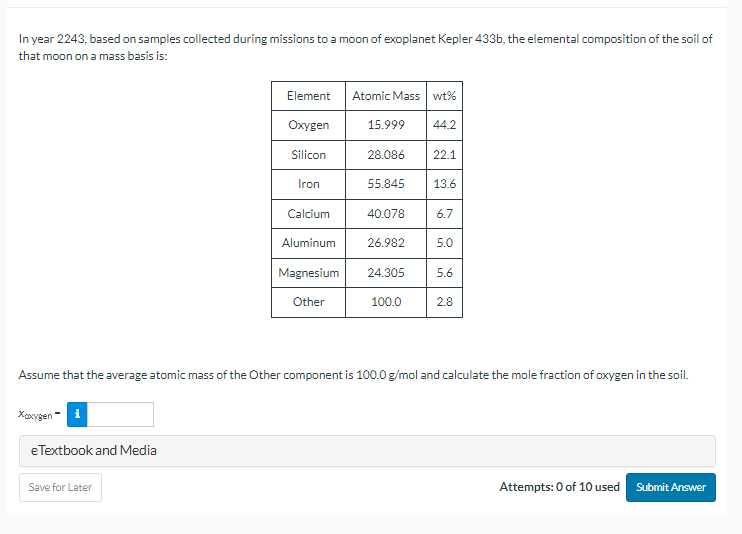
In year based on samples collected during missions to a moon of exoplanet Kepler the elemental composition of the soil of
that moon on a mass basis is:
Assume that the average atomic mass of the Other component is and calculate the mole fraction of oxygen in the soil.
eTextbook and Media
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


