Question: In year 2284, based on samples collected during missions to a moon of exoplanet Kepler 433b, the elemental composition of the soil of that moon
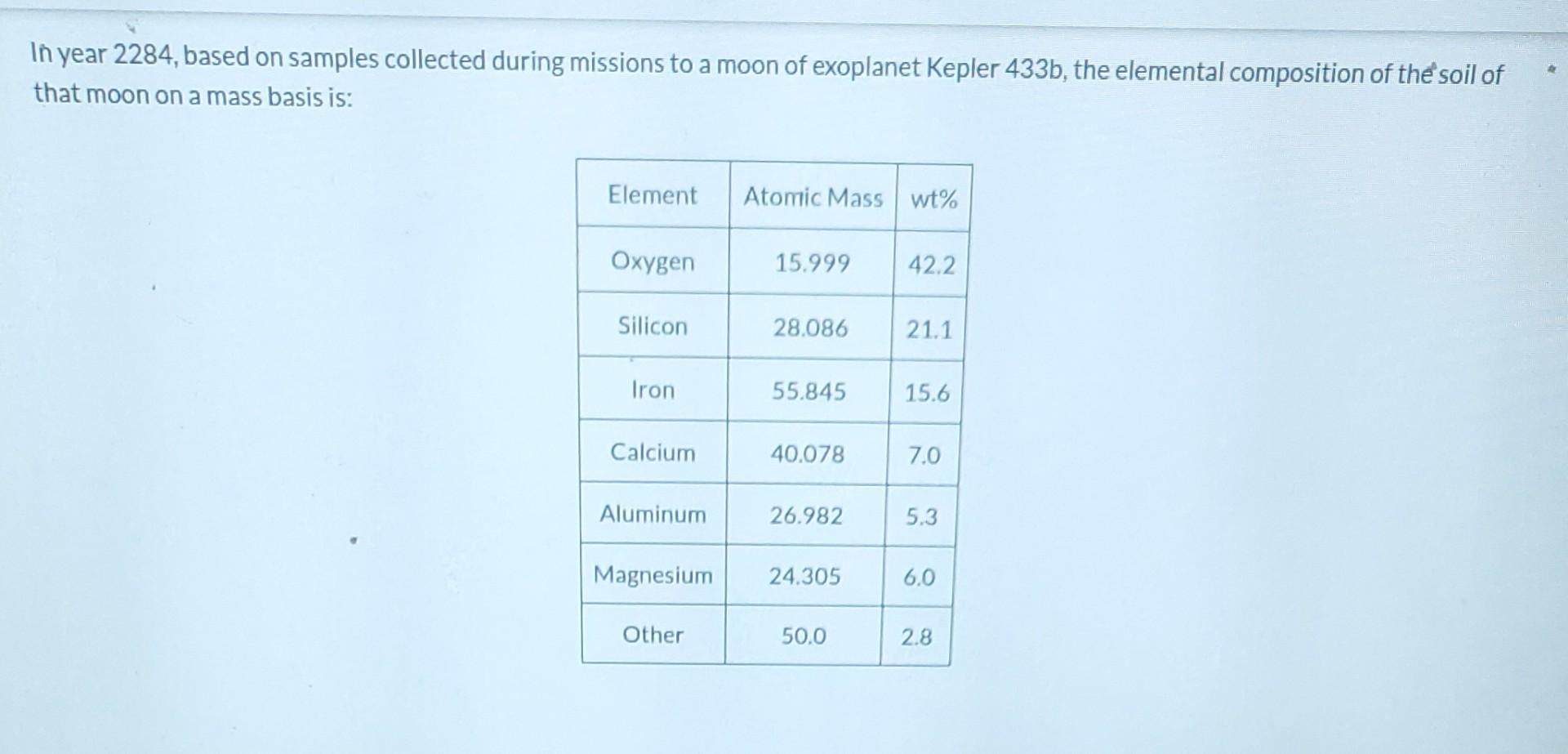

In year 2284, based on samples collected during missions to a moon of exoplanet Kepler 433b, the elemental composition of the soil of that moon on a mass basis is: Element Atomic Mass wt% Oxygen 15.999 42.2 Silicon 28.086 21.1 Iron 55.845 15.6 Calcium 40.078 7.0 Aluminum 26.982 5.3 Magnesium 24.305 6.0 Other 50.0 2.8 3.3 Magnesium 24.305 6.0 Other 50.0 2.8 Assume that the average atomic mass of the Other component is 50.0 g/mol and calculate the mole fraction of oxygen in the soil. Xoxygen
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


