Question: Incorrect Your answer is incorrect. - Row 1: Your answer is incorrect. - Row 2: Your answer is incorrect. Some measurements of the initial rate
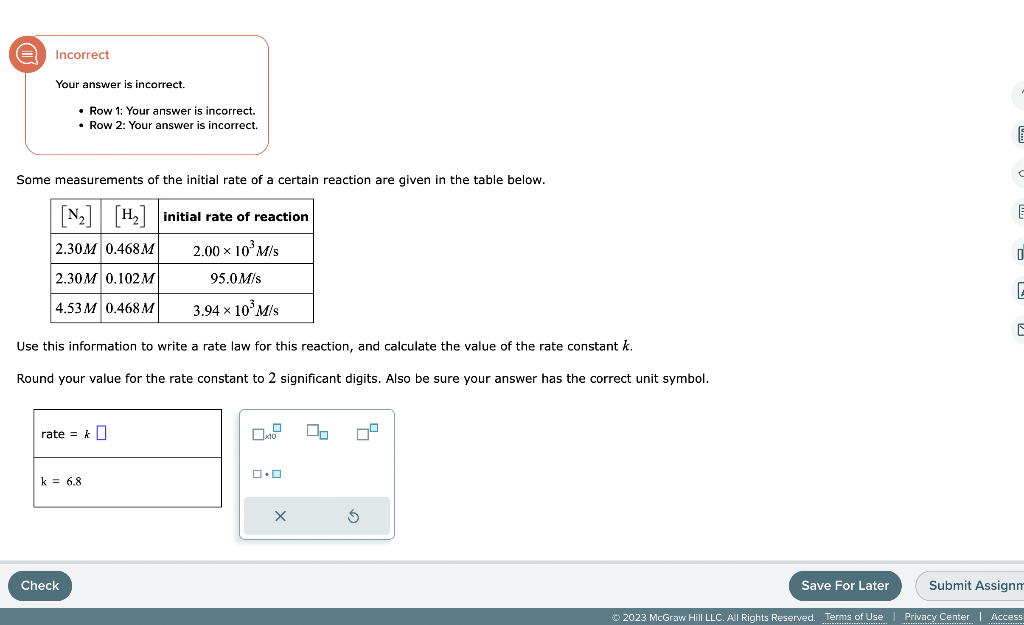
Incorrect Your answer is incorrect. - Row 1: Your answer is incorrect. - Row 2: Your answer is incorrect. Some measurements of the initial rate of a certain reaction are given in the table below. Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 2 significant digits. Also be sure your answer has the correct unit symbol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


