Question: HELP !!!!!!!!! PLEASE HELP ME ON THE RED MARK CALCULATION. BE SURE UPLOAD THE GRAPH. make sure Answer all of them Experiment 1 Exact concentration
HELP !!!!!!!!! PLEASE HELP ME ON THE RED MARK CALCULATION. BE SURE UPLOAD THE GRAPH. make sure Answer all of them
Experiment 1
Exact concentration of H2O2 stock solution (M)
1.014
Temperature of water bath (C)
24
Experiment 1: Volume of O2 vs time for decomposition of H2O2
| Time (min) | |
|---|---|
| Volume of O2 (mL) 2.0 | 0.00 |
| 4 | 1.22 |
| 6 | 2.41 |
| 8 | 3.64 |
| 10 | 4.93 |
| 12 | 6.34 |
| 14 | 7.78 |
| 16 | 9.49 |
| 18 | 10.99 |
| 20 | 12.35 |
| 22 | 14.64 |
| 24 | 16.31 |
| 26 | 19.16 |
| 28 | 20.97 |
| 30 | 24.52 |
| 32 | 26.03 |
| 34 | 28.99 |
| 36 | 32.30 |
| 38 | 35.17 |
| 40 | 38.83 |
| 42 | 44.83 |
| 44 | 50.50 |
| 46 | 58.26 |
| 48 | 63.46 |
Experiment 2: Volume of O2 vs time for decomposition of H2O2
| Time (min) | |
|---|---|
| Volume of O2 (mL) 2.0 | 0.00 |
| 4 | 0.63 |
| 6 | 1.31 |
| 8 | 1.96 |
| 10 | 2.42 |
| 12 | 2.93 |
| 14 | 3.56 |
.
Experiment 3: Volume of O2 vs time for decomposition of H2O2
| Time (min) | |
|---|---|
| Volume of O2 (mL) 2.0 | 0.00 |
| 4 | 0.45 |
| 6 | 0.88 |
| 8 | 1.44 |
| 10 | 2.29 |
| 12 | 2.92 |
| 14 | 3.51 |
(24pts) Calculations
For all three experiments plot the volume of O2 evolved in mL (on the y-axis) versus time in minutes (on the x-axis). Clearly label each experiment's plot and/or use a different color for each. Determine each initial rate of reaction (slope of the tangent at the beginning of the reaction). Zero time corresponds to the first volume reading at 2.0 mLyou must have entered your data this way during data entry. Label your axes so that your actual plotting encompasses at least half of your graph paper in each direction.
Experiment 1
(0.5pts)
What is the last point that you will use to determine the initial rate?
Choose...4.0 mL6.0 mL8.0 mL10.0 mL12.0 mL14.0 mL16.0 mL18.0 mL20.0 mL22.0 mL24.0 mL26.0 mL28.0 mL30.0 mL32.0 mL34.0 mL36.0 mL38.0 mL40.0 mL42.0 mL44.0 mL46.0 mL48.0 mL
(0.5pts)
Slope of tangent line for experiment 1:
Experiment 2
(0.5pts)
What is the last point that you will use to determine the initial rate?
Choose...4.0 mL6.0 mL8.0 mL10.0 mL12.0 mL14.0 mL
(0.5pts)
Slope of tangent line for experiment 2:
Experiment 3
(0.5pts)
What is the last point that you will use to determine the initial rate?
Choose...4.0 mL6.0 mL8.0 mL10.0 mL12.0 mL14.0 mL
(0.5pts)
Slope of tangent line for experiment 3:
(2pts)
Upload the graph(s) of your data here. Make sure each plot is properly labeled (axes defined, units labeled, titled properly). You can plot all three experiments on one graph, or split into three graphs.
Browse your files to upload
or Drag and Drop
Concentration of H2O2 stock solution: 1.014 M
Concentration of KI stock solution: 0.10 M
Orders of reaction and specific rate constant
Table view
List view
Orders of reaction and specific rate constant
| Experiment 1 | Experiment 2 | Experiment 3 | |
|---|---|---|---|
| Initial molarity of H2O2 after dilution (M) | |||
| Initial molarity of I- after dilution (M) | |||
| Initial rate of reaction (mL O2/min) (slope of tangent determined above) | |||
| Order of reaction with respect to H2O2 (estimated) | --------- (blank) |
| |
| Order of reaction with respect to I- (estimated) | --------- (blank) |
| |
| Order of reaction with respect to H2O2 (calculated) | |||
| Order of reaction with respect to I- (calculated) | |||
| Specific rate constant (using the calculated reaction order) | |||
| Average specific rate constant |
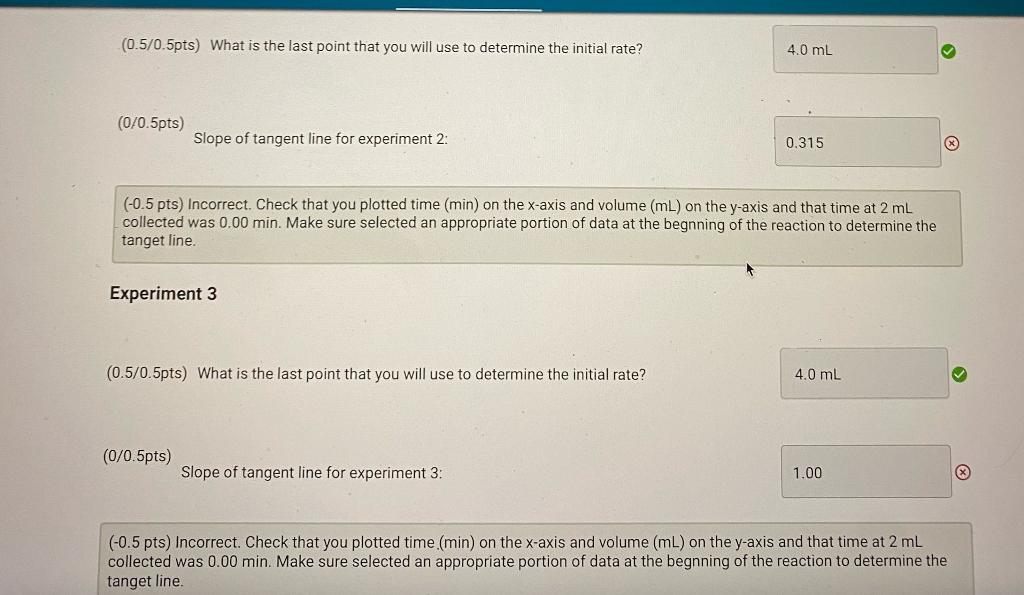
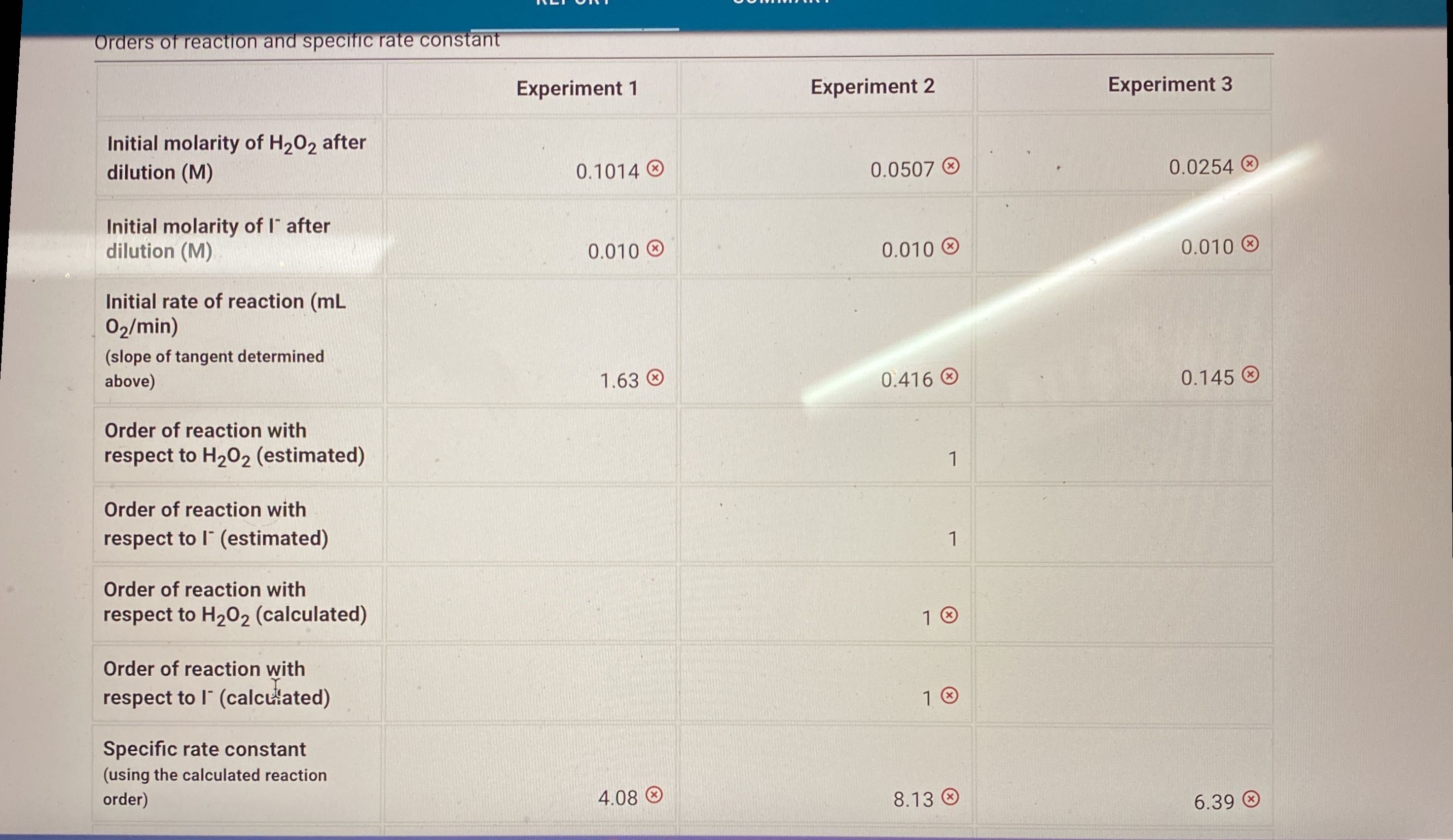
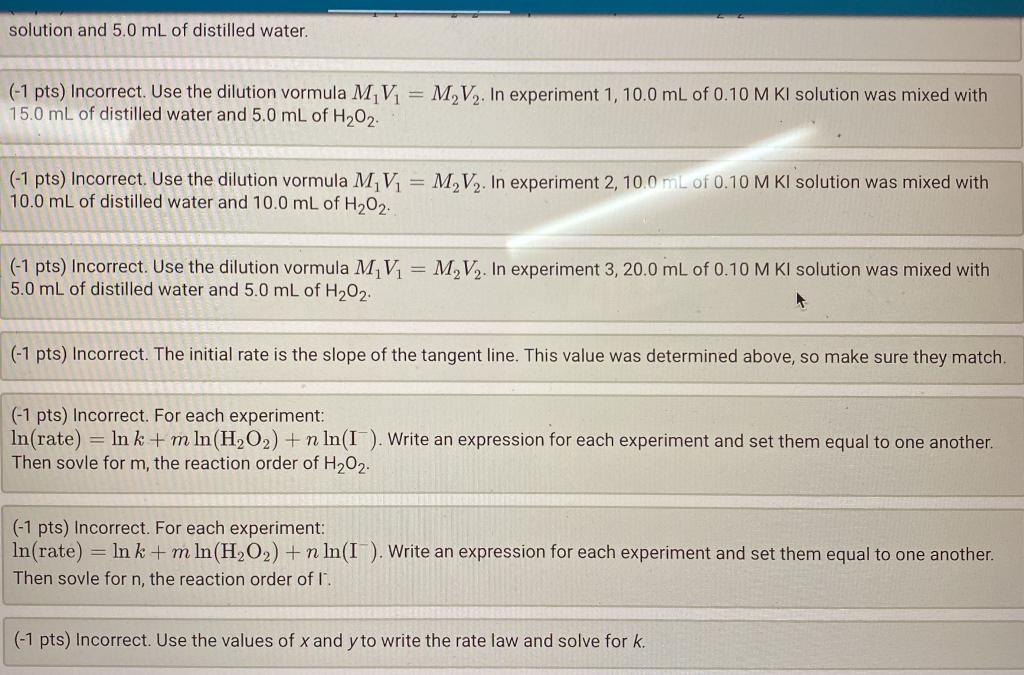
(0.5/0.5pts) What is the last point that you will use to determine the initial rate? (0/0.5pts) Slope of tangent line for experiment 2 : ( 0.5 pts) Incorrect. Check that you plotted time (min) on the x-axis and volume (mL) on the y-axis and that time at 2mL collected was 0.00min. Make sure selected an appropriate portion of data at the begnning of the reaction to determine the tanget line. Experiment 3 (0.5/0.5pts) What is the last point that you will use to determine the initial rate? (0/0.5pts) Slope of tangent line for experiment 3: (0.5pts) Incorrect. Check that you plotted time. (min) on the x-axis and volume (mL) on the y-axis and that time at 2mL collected was 0.00min. Make sure selected an appropriate portion of data at the begnning of the reaction to determine the tanget line. Orders of reaction and specific rate constant Experiment 1 Experiment 2 Experiment 3 Initial molarity of H2O2 after dilution (M) 0.1014 0.0507 0.0254 Initial molarity of Iafter dilution (M) Initial rate of reaction (mL O2/min) (slope of tangent determined above) 1.63 0.416 0.145 Order of reaction with respect to H2O2 (estimated) 1 Order of reaction with respect to I(estimated) 1 Order of reaction with respect to H2O2 (calculated) 1 Order of reaction with respect to I(calcuisated) 1 Specific rate constant (using the calculated reaction order) 4.08 8.13 6.39 solution and 5.0mL of distilled water. (-1 pts) Incorrect. Use the dilution vormula M1V1=M2V2. In experiment 1,10.0mL of 0.10MKI solution was mixed with 15.0mL of distilled water and 5.0mL of H2O2 (-1 pts) Incorrect. Use the dilution vormula M1V1=M2V2. In experiment 2,10.0mL of 0.10MKI solution was mixed with 10.0mL of distilled water and 10.0mL of H2O2 (-1 pts) Incorrect. Use the dilution vormula M1V1=M2V2. In experiment 3,20.0mL of 0.10MKI solution was mixed with 5.0mL of distilled water and 5.0mL of H2O2. (- 1 pts) Incorrect. The initial rate is the slope of the tangent line. This value was determined above, so make sure they match. (-1 pts) Incorrect. For each experiment: ln( rate )=lnk+mln(H2O2)+nln(I). Write an expression for each experiment and set them equal to one another. Then sovle for m, the reaction order of H2O2. (-1 pts) Incorrect. For each experiment: ln( rate )=lnk+mln(H2O2)+nln(I). Write an expression for each experiment and set them equal to one another. Then sovle for n, the reaction order I. (- 1 pts) Incorrect. Use the values of x and y to write the rate law and solve for k
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


