Question: Indicate whether fluid A is in equilibrium with fluid B (fluid B has a molecular weight of 18g/gmol). Consider that both are subjected to a
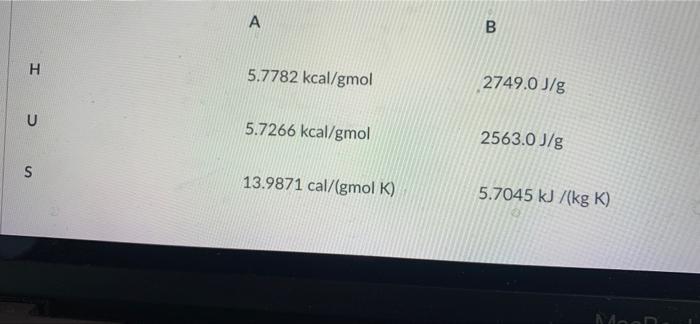
A B H 5.7782 kcal/gmol 2749.0 J/g U 5.7266 kcal/gmol 2563.0 J/g S 13.9871 cal/(gmol K) 5.7045 kJ/(kg K) More
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


