Question: Indicate whether you think the following molecules would be lower energy if they were completely planar (all atoms sp2 hybridized) or partially non-planar (at least
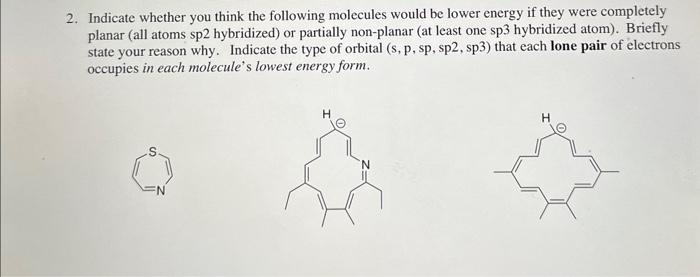
Indicate whether you think the following molecules would be lower energy if they were completely planar (all atoms sp2 hybridized) or partially non-planar (at least one sp3 hybridized atom). Briefly state your reason why. Indicate the type of orbital (s,p,sp, sp2, sp3) that each lone pair of electrons occupies in each molecule's lowest energy form
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


