Question: Information: A base is any substance that seeks a full () or partial (8) positive charge on a HYDROGEN. Hydrogen atoms are usually on the
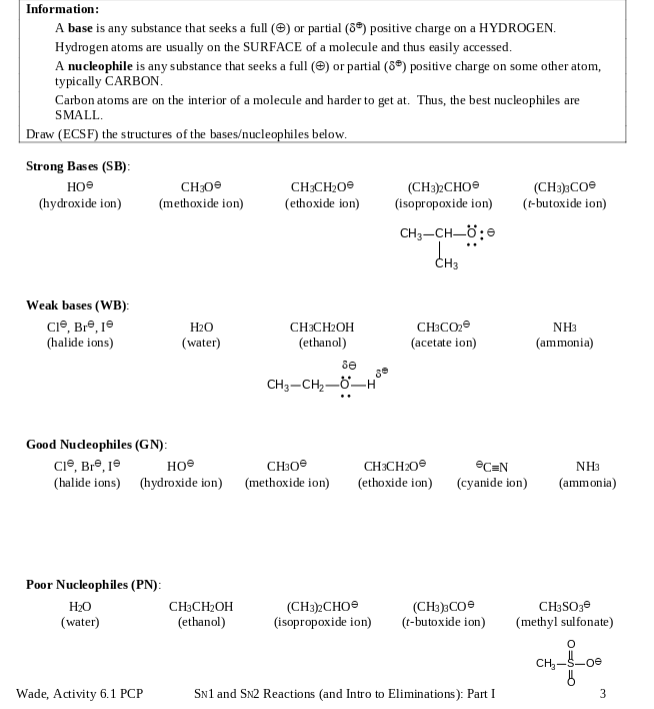
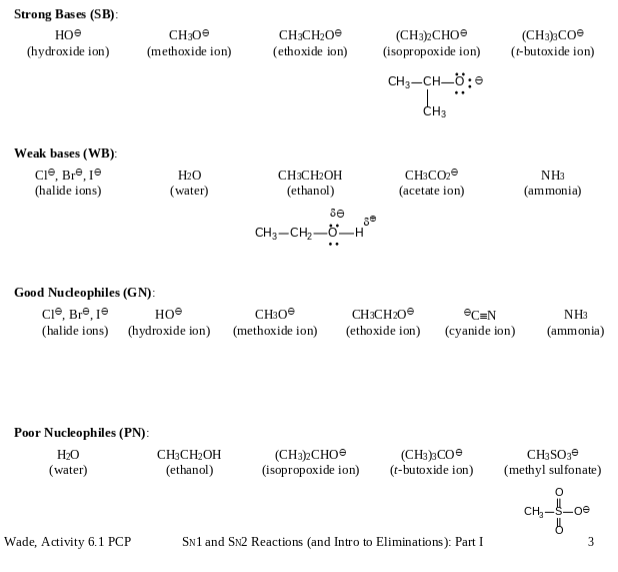
Information: A base is any substance that seeks a full () or partial (8) positive charge on a HYDROGEN. Hydrogen atoms are usually on the SURFACE of a molecule and thus easily accessed. A nucleophile is any substance that seeks a full () or partial (8) positive charge on some other atom, typically CARBON. Carbon atoms are on the interior of a molecule and harder to get at. Thus, the best nucleophiles are SMALL. Draw (ECSF) the structures of the basesucleophiles below. Strong Bases (SB): CH300 CH3CHO (CH3)2CHO (isopropoxide ion) (hydroxide ion) (methoxide ion) (ethoxide ion) (CH3)3CO (t-butoxide ion) CHCH_;8 CH Weak bases (WB): CI, Bre, 10 HO (water) CH3CH2OH (ethanol) CH3CO (acetate ion) (halide ions) NH3 (ammonia) se CH3CH,_H Good Nucleophiles (GN): Cl, Bre, 1 CH3O (halide ions) (hydroxide ion) (methoxide ion) CH3CHOH (ethanol) (CH3)2CHO (isopropoxide ion) (CH3)3CO (t-butoxide ion) SN1 and SN2 Reactions (and Intro to Eliminations): Part I Poor Nucleophiles (PN): HO (water) Wade, Activity 6.1 PCP CH3CH2O (ethoxide ion) eC=N (cyanide ion) NH3 (ammonia) CH3SO3 (methyl sulfonate) O CH-5-00 3 Strong Bases (SB) e (hydroxide ion) CH30 (methoxide ion) CH3CH200 (ethoxide ion) (CH3)3CO (t-butoxide ion) (CH3)2CHO (isopropoxide ion) CH3-CH=0; dhs Weak bases (WB) CI, Bre, je (halide ions) H2O (water) CH3C02 (acetate ion) NH3 (ammonia) CH3CH2OH (ethanol) se CH3 -CH2--H Good Nudeophiles (GN): Cl, Br, je CH (halide ions) (hydroxide ion) (methoxide ion) CH3CH20 (ethoxide ion) CEN (cyanide ion) NH3 (ammonia) Poor Nucleophiles (PN): H20 CH3CH2OH (water) (ethanol) (CH3)2CHO (isopropoxide ion) (CH3)3CO (t-butoxide ion) CH3SO3e (methyl sulfonate) CH oe 6 3 Wade, Activity 6.1 PCP Sn1 and SN2 Reactions and Intro to Eliminations): Part
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


