Question: Initial rate data at a certain temperature is given in the table for the following reaction. The rase law has the form rate=[NOBr]2 where x
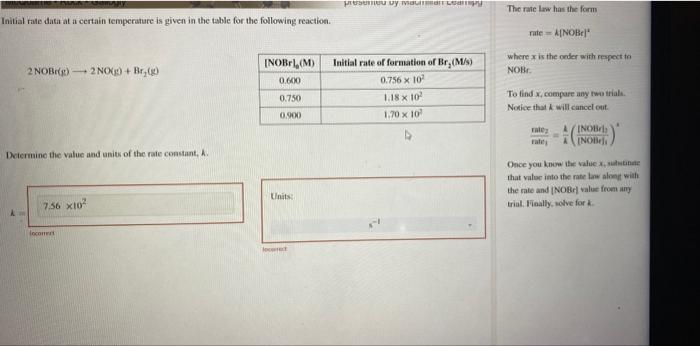
Initial rate data at a certain temperature is given in the table for the following reaction. The rase law has the form rate=[NOBr]2 where x is the ceder with reqpect to 2NOBr(r)2NO(g)+Br2(g) Nofir. To lind x, compore any twe trials. Notice that k will cancel out. Determine the value and anits of the rate constant, h. Once you know the value x, riblutiate that valoe iato the rate las alnegf wilh the mate and [NOBr] value froet anf trial. Faally, wolve for k
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


