Question: Initially, 3.50molN2O4 were placed in an empty 2.00L container. After equilibrium was established, it was found that 25.0% of the N2O4 had decomposed according to
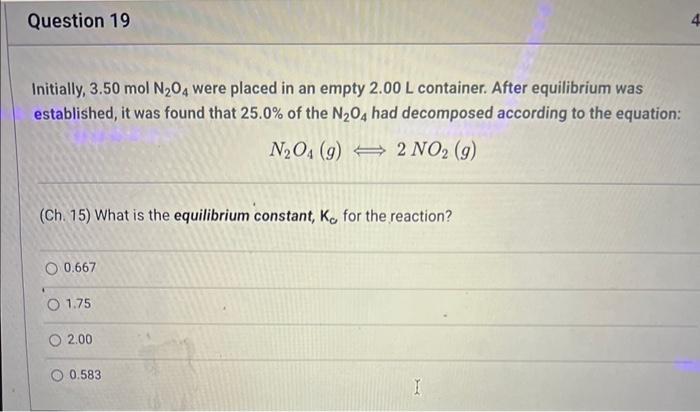
Initially, 3.50molN2O4 were placed in an empty 2.00L container. After equilibrium was established, it was found that 25.0% of the N2O4 had decomposed according to the equation: N2O4(g)2NO2(g) (Ch. 15) What is the equilibrium constant, Kc for the reaction? 0.667 1.75 2.00 0.583
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


