Question: Initial-rate data at a certain temperature is given in the table for the reaction SO2Cl2(g)SO2(g)+Cl2(g) Determine the value and units of the rate constant. k=
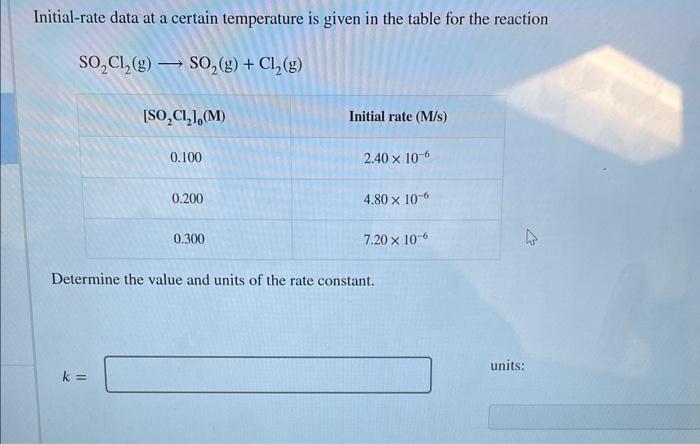
Initial-rate data at a certain temperature is given in the table for the reaction SO2Cl2(g)SO2(g)+Cl2(g) Determine the value and units of the rate constant. k= units
Step by Step Solution
There are 3 Steps involved in it
To determine the value and units of the rate constant k for the reaction textSO2textCl2 ightarrow te... View full answer

Get step-by-step solutions from verified subject matter experts


