Question: Instructions: Draw the partial charges (+ and ) and direction (dipole moment of the more electronegative atom. Then, indicate if the molecule is polar or
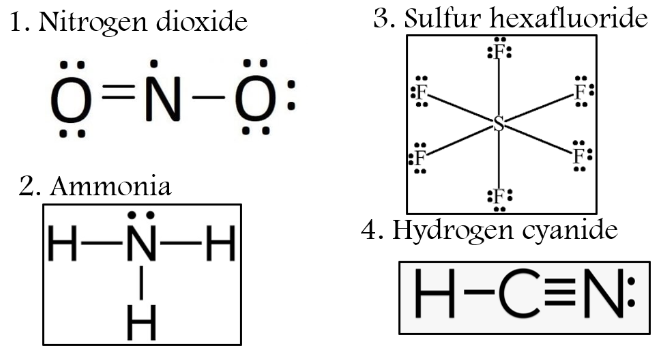
Instructions: Draw the partial charges (+ and ) and direction (dipole moment of the more electronegative atom. Then, indicate if the molecule is polar or nonpolar. 1. Nitrogen dioxide 3. Sulfur hexafluoride O=NO: 2. Ammonia 4. Hydrogen cyanide
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


