Question: Instructions: Solve the following problem, showing all the steps you made in the procedure. The solution must be made in four pages (maximum) including the
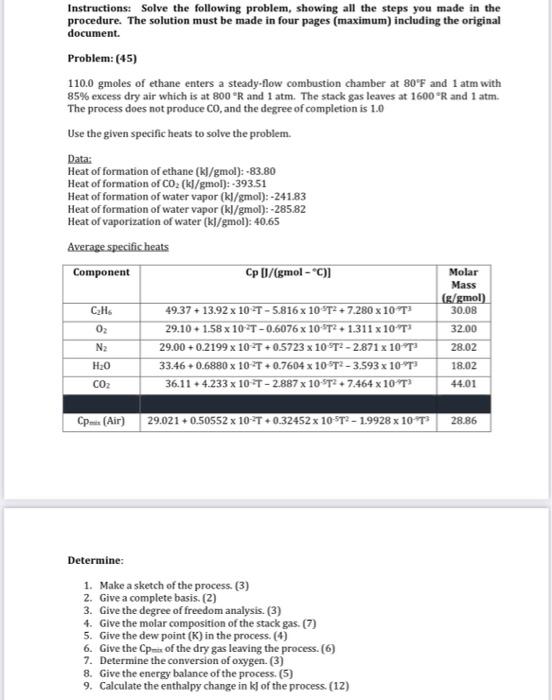
Instructions: Solve the following problem, showing all the steps you made in the procedure. The solution must be made in four pages (maximum) including the original document. Problem: (45) 110.0 gmoles of ethane enters a steady-flow combustion chamber at 80'F and 1 atm with 85% excess dry air which is at 800R and 1 atm. The stack gas leaves at 1600R and 1 atm. The process does not produce Co, and the degree of completion is 1.0 Use the given specific heats to solve the problem. Data: Heat of formation of ethane (kJ/gmol): -83.80 Heat of formation of CO2 (kJ/gmol): -393.51 Heat of formation of water vapor (kl/gmol): -241.83 Heat of formation of water vapor (kl/gmol): -285.82 Heat of vaporization of water (kl/gmol): 40.65 Average specificheats Component Cp U/(gmol - C) (g/gmol) C.HS 49.37 + 13.92 x 10T -5.816 x 10T2 + 7.280 x 10T 30.08 02 29.10 + 1.58 x 102T-0.6076 x 10-T2 + 1.311 x 10 T 32.00 N2 29.00 +0.2199 x 10-T+0.5723 x 10 T- - 2.871x10 T 28.02 33.46 +0.6880 x 10-2T+0.7604 x 10 ST2-3.593 x 10-13 CO2 36.11 4.233 x 10- T -2.887x10-ST2 + 7.464 x 10T 44.01 Molar Mass HO 18.02 CP (Air) 29.021 +0.50552 x 10-T+0.32452 x 10ST - 1.9928 x 10T* 28.86 Determine: 1. Make a sketch of the process. (3) 2. Give a complete basis. (2) 3. Give the degree of freedom analysis. (3) 4. Give the molar composition of the stack gas. (7) 5. Give the dew point (K) in the process. (4) 6. Give the Come of the dry gas leaving the process. (6) 7. Determine the conversion of oxygen. (3) 8. Give the energy balance of the process. (5) 9. Calculate the enthalpy change in kl of the process (12)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


