Question: Instrumental Analysis Please explain the answer for me! The red lines are the answer for this problem. But I don't understand. Please help explain the
Instrumental Analysis
Please explain the answer for me! The red lines are the answer for this problem. But I don't understand. Please help explain the red lines are highlighted with yellow!
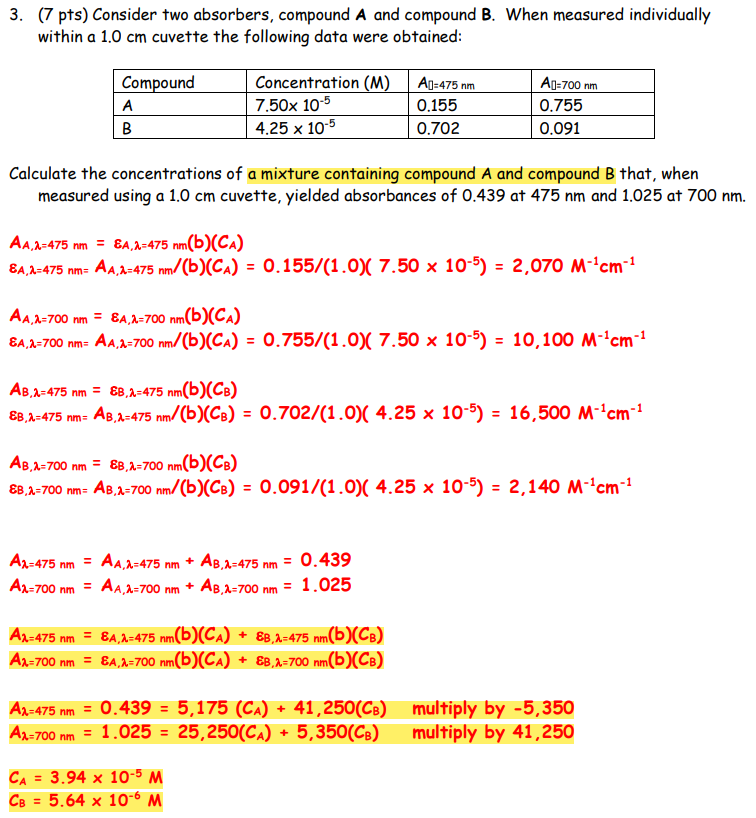
3. (7 pts) Consider two absorbers, compound A and compound B. When measured individually within a 1.0cm cuvette the following data were obtained: Calculate the concentrations of a mixture containing compound A and compound B that, when measured using a 1.0cm cuvette, yielded absorbances of 0.439 at 475nm and 1.025at700nm AA,=475nm=A,=475nm(b)(CA)A,=475nm=AA,=475nm/(b)(CA)=0.155/(1.0)(7.50105)=2,070M1cm1AA,=700nm=A,=700nm(b)(CA)A,=700nm=AA,=700nm/(b)(CA)=0.755/(1.0)(7.50105)=10,100M1cm1 AB,=475nm=B,=475nm(b)(CB) B,=475nm=AB,=475nm/(b)(CB)=0.702/(1.0)(4.25105)=16,500M1cm1 AB,=700nm=B,=700nm(b)(CB) B,=700nm=AB,=700nm/(b)(CB)=0.091/(1.0)(4.25105)=2,140M1cm1 A=475nm=AA,=475nm+AB,=475nm=0.439A=700nm=AA,=700nm+AB,=700nm=1.025 A=475nm=A,=475nm(b)(CA)+B,=475nm(b)(CB)A=700nm=A,=700nm(b)(CA)+B,=700nm(b)(CB) A=475nm=0.439=5,175(CA)+41,250(CB)A=700nmmultiplyby5,350=1.025=25,250(CA)+5,350(CB)multiplyby41,250 CA=3.94105MCB=5.64106M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


