Question: Intermolecular forces are the interactions between molecules and are generally weaker than bonds within nolecules Or these choices, which molecule will have dipole-digole forces with
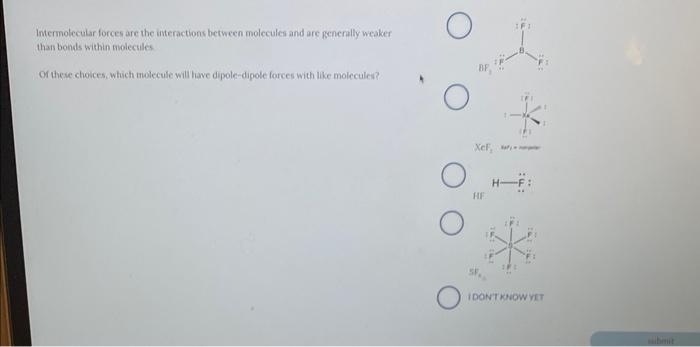

Intermolecular forces are the interactions between molecules and are generally weaker than bonds within nolecules Or these choices, which molecule will have dipole-digole forces with like molecules? 1. What are the physical properties (including formula weight, melting point and water solubility) and hazards associated with alum, KAl(SO4)212H2O ? (Use a MSDS source for this data.) 2. You started your preparation of common alum with 0.9156g of aluminum foil. a. What is the theoretical yield of alum starting with this much aluminum? (Show work) b. You isolated 8.3181g of alum. What was the percent yield of your product? (Show work)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


