Question: IS es I needed for this question. 1 pts 2req 1 pts 2reg According to the ideal gas law, a 1.021 mol sample of argon
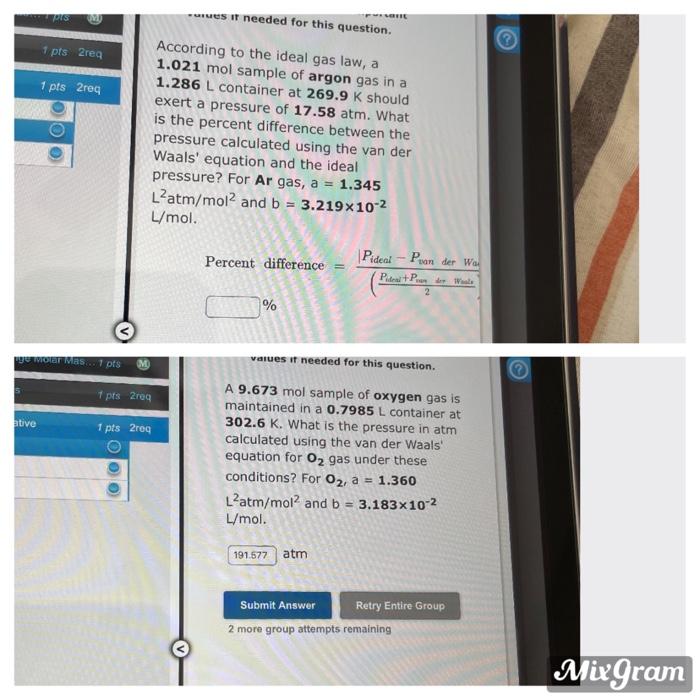
IS es I needed for this question. 1 pts 2req 1 pts 2reg According to the ideal gas law, a 1.021 mol sample of argon gas in a 1.286 L container at 269.9 K should exert a pressure of 17.58 atm. What is the percent difference between the pressure calculated using the van der Waals' equation and the ideal pressure? For Ar gas, a = 1.345 L'atm/mol and b = 3.219x10-2 L/mol. Pideal - Puan der Wa Percent difference = P.PL 2 % We Wer was 1 pts M 7 prs2rea ative 1 pts 2reg values if needed for this question. A 9.673 mol sample of oxygen gas is maintained in a 0.7985 L container at 302.6 K. What is the pressure in atm calculated using the van der Waals equation for O2 gas under these conditions? For O2, a = 1.360 Llatm/moland b = 3.183x10-2 L/mol. 191,677 atm Submit Answer Retry Entire Group 2 more group attempts remaining Mixgram
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


