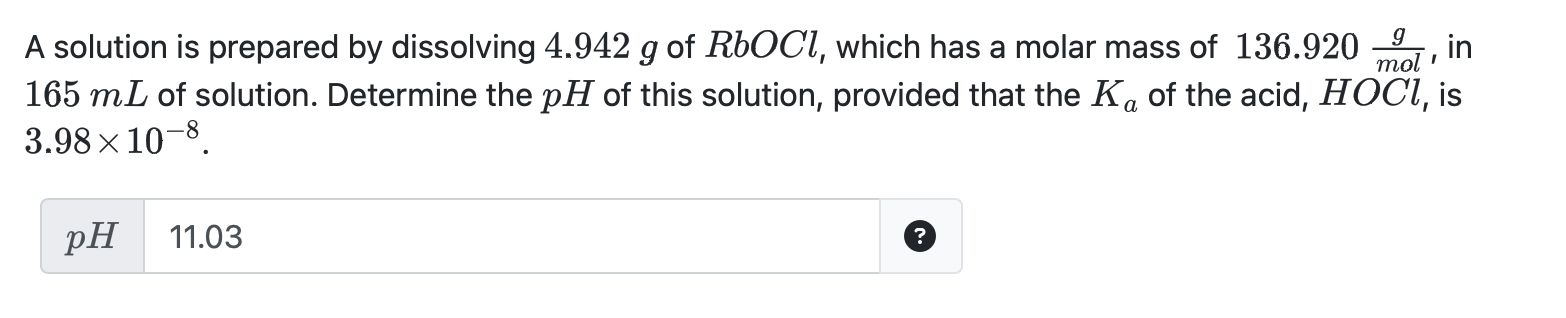
Question: Is this answer right? A solution is prepared by dissolving 4.942g of RbOCl, which has a molar mass of 136.920molg, in 165mL of solution. Determine
 Is this answer right?
Is this answer right?
A solution is prepared by dissolving 4.942g of RbOCl, which has a molar mass of 136.920molg, in 165mL of solution. Determine the pH of this solution, provided that the Ka of the acid, HOCl, is 3.98108
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


