Question: *Isomers are different molecules with the same formula. C2H2Cl2 is the formula of three different molecules. Make sure you draw structures for the three isomers.
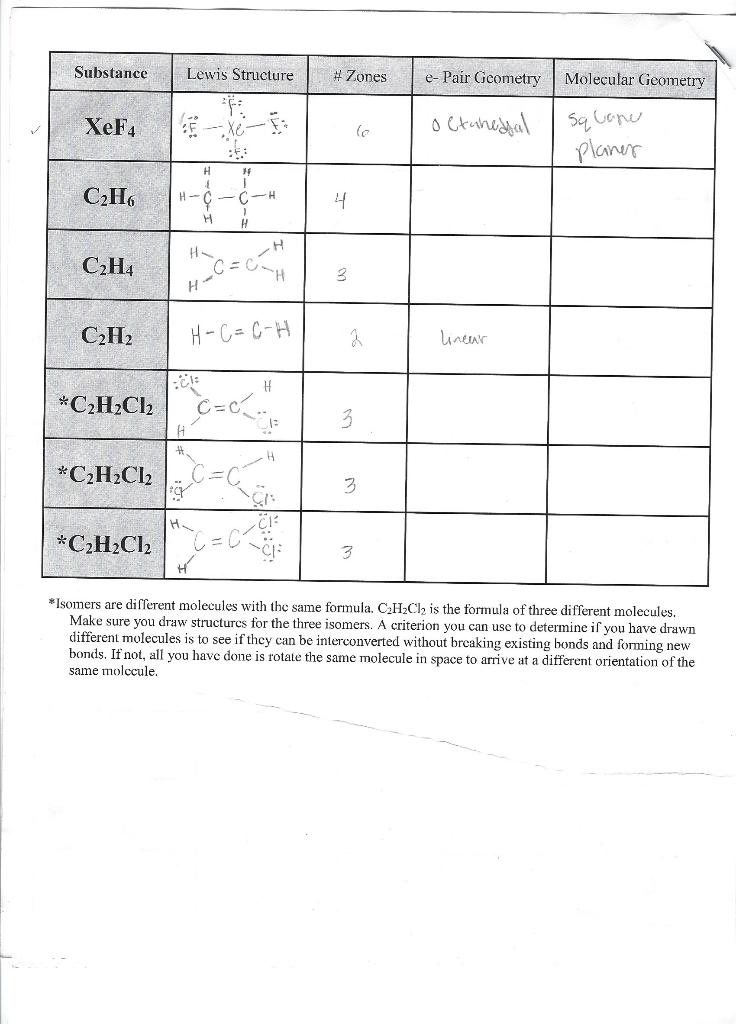
*Isomers are different molecules with the same formula. C2H2Cl2 is the formula of three different molecules. Make sure you draw structures for the three isomers. A criterion you can use to determine if you have drawn different molecules is to see if they can be interconverted without breaking existing bonds and forming new bonds. If not, all you have done is rotate the same molecule in space to arrive at a different orientation of the same molecule
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


