Question: Isooctane and air react as shown below: C8H18 +10(02 +3.76 N2) 4 CO2 + 4 CO +8 H2O + H2 + 37.6 N2 If
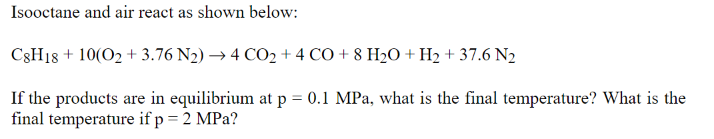
Isooctane and air react as shown below: C8H18 +10(02 +3.76 N2) 4 CO2 + 4 CO +8 H2O + H2 + 37.6 N2 If the products are in equilibrium at p = 0.1 MPa, what is the final temperature? What is the final temperature if p = 2 MPa?
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


