Question: it is a physicochemistry question 1.Consider the below reaction 2A+BC+D with rA=kCACB where CA and CB show concentrations of reactant A and B. When CA0=0.024mol/L
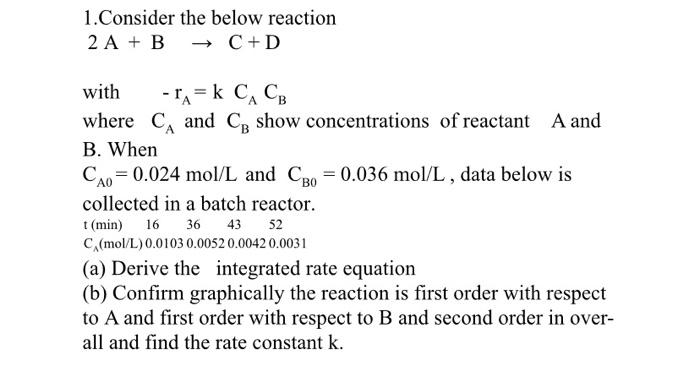
1.Consider the below reaction 2A+BC+D with rA=kCACB where CA and CB show concentrations of reactant A and B. When CA0=0.024mol/L and CB0=0.036mol/L, data below is collected in a batch reactor. (a) Derive the integrated rate equation (b) Confirm graphically the reaction is first order with respect to A and first order with respect to B and second order in overall and find the rate constant k
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


