Question: It is necessary to design a packed-bed reactor (PBR) for the gas-phase irreversible hydrogenation of acetylene (A) to produce ethylene (E): C2H2+H2C2H4 a) Consider the
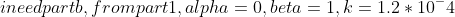
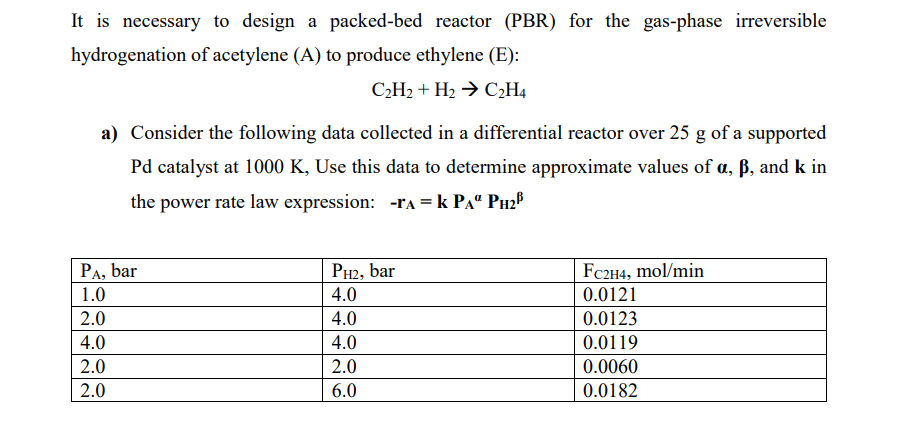

It is necessary to design a packed-bed reactor (PBR) for the gas-phase irreversible hydrogenation of acetylene (A) to produce ethylene (E): C2H2+H2C2H4 a) Consider the following data collected in a differential reactor over 25g of a supported Pd catalyst at 1000K, Use this data to determine approximate values of ,, and k in the power rate law expression: rA=kPAPH2 For a 1000L/min gas stream containing equimolar quantities of A and H2 and at 10atm and 400K, use power rate law derived in (a) to determine the catalyst mass required for 70% conversion of A in a PBR. Assume that the pressure drop is negligible and that the reaction is isothermal [CEG, 2018]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


