Question: ive been having a very difficult time with this study packet. if someone could please help me out with all of the problems that would
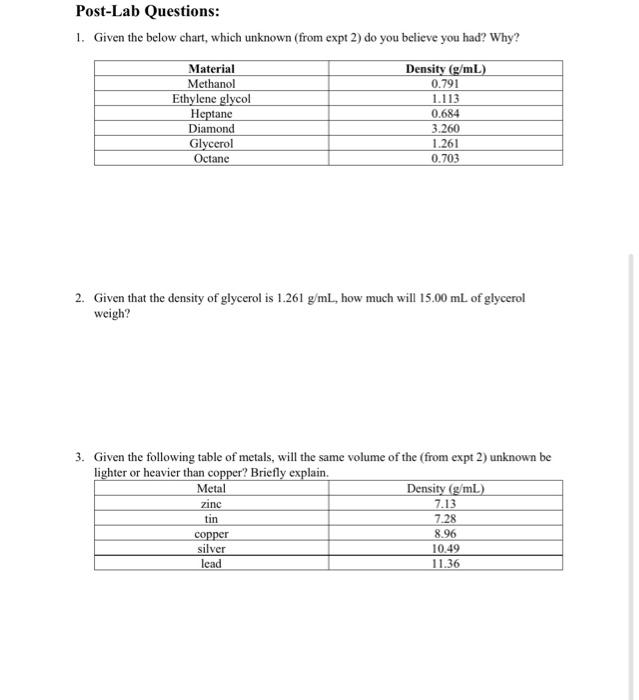


Post-Lab Questions: 1. Given the below chart, which unknown (from expt 2) do you believe you had? Why? Material Methanol Ethylene glycol Heptane Diamond Glycerol Octane Density (g/mL) 0.791 1.113 0.684 3.260 1.261 0.703 2. Given that the density of glycerol is 1.261 g/mL, how much will 15.00 mL of glycerol weigh? 3. Given the following table of metals, will the same volume of the (from expt 2) unknown be lighter or heavier than copper? Briefly explain. Metal Density (g/mL) zinc 7.13 tin 7.28 copper 8.96 silver 10.49 lead 11.36 Experiment 2: Identify an Unknown ????? Unknown ?????? 1. Mass of dry unknown (gram) 2. Initial volume of water without unknown (milliliter) 3. Final volume of water with unknown (milliliter) 4. Volume of unknown (milliliter) 5. Density of unknown (gram milliliter) Show workings for item 4 above here. Show workings for item 5 above here: Density Measurement Lab Experiment 1: Find the Density of Various Metals Iron Gold Lead 1. Mass of dry metal (gram) 2. Initial volume of water without metal (milliliter) 3. Final volume of water with metal (milliliter) 4. Volume of metal (milliliter) 5. Density of metal (gram/milliliter) Show workings for item 4 above here: Show workings for item 5 above here: Post-Lab Questions: 1. Given the below chart, which unknown (from expt 2) do you believe you had? Why? Material Methanol Ethylene glycol Heptane Diamond Glycerol Octane Density (g/mL) 0.791 1.113 0.684 3.260 1.261 0.703 2. Given that the density of glycerol is 1.261 g/mL, how much will 15.00 mL of glycerol weigh? 3. Given the following table of metals, will the same volume of the (from expt 2) unknown be lighter or heavier than copper? Briefly explain. Metal Density (g/mL) zinc 7.13 tin 7.28 copper 8.96 silver 10.49 lead 11.36 Experiment 2: Identify an Unknown ????? Unknown ?????? 1. Mass of dry unknown (gram) 2. Initial volume of water without unknown (milliliter) 3. Final volume of water with unknown (milliliter) 4. Volume of unknown (milliliter) 5. Density of unknown (gram milliliter) Show workings for item 4 above here. Show workings for item 5 above here: Density Measurement Lab Experiment 1: Find the Density of Various Metals Iron Gold Lead 1. Mass of dry metal (gram) 2. Initial volume of water without metal (milliliter) 3. Final volume of water with metal (milliliter) 4. Volume of metal (milliliter) 5. Density of metal (gram/milliliter) Show workings for item 4 above here: Show workings for item 5 above here
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


