Question: I've solved the first question but I'm not sure how to solve the second question about actual concentration. Please help me with it. 1. Calculate
I've solved the first question but I'm not sure how to solve the second question about actual concentration. Please help me with it.
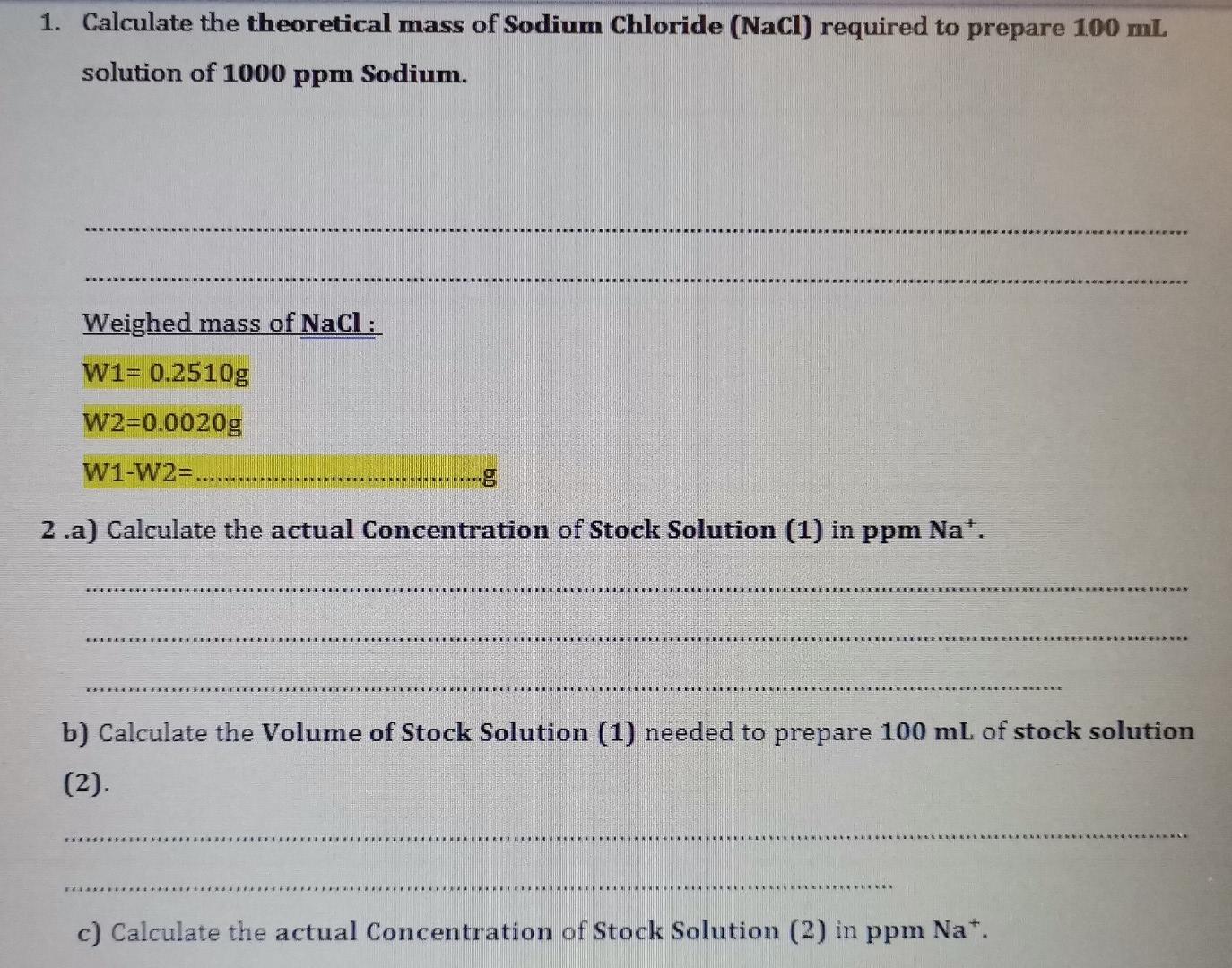
1. Calculate the theoretical mass of Sodium Chloride (NaCl) required to prepare 100 ml solution of 1000 ppm Sodium. Weighed mass of NaCl : W1= 0.2510g W2=0.0020g W1-W2=...... 2.a) Calculate the actual Concentration of Stock Solution (1) in ppm Na*. b) Calculate the Volume of Stock Solution (1) needed to prepare 100 mL of stock solution (2). c) Calculate the actual Concentration of Stock Solution (2) in ppm Na*
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


