Question: J Chapter = 4.18x103 Q = mcAT Q = ml kg . C Specific Heat of Ice 2090 J kg-1 K-1 Specific Heat of Water
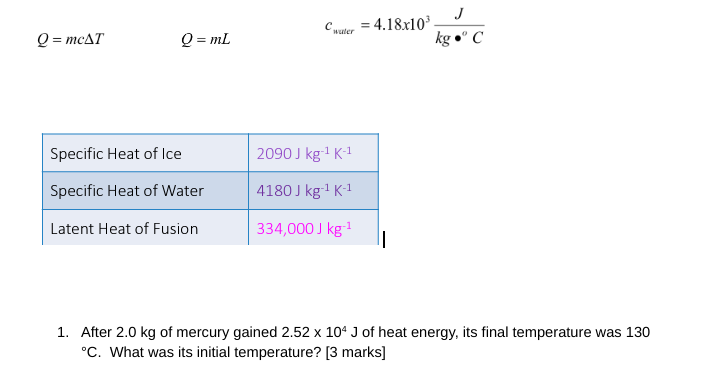

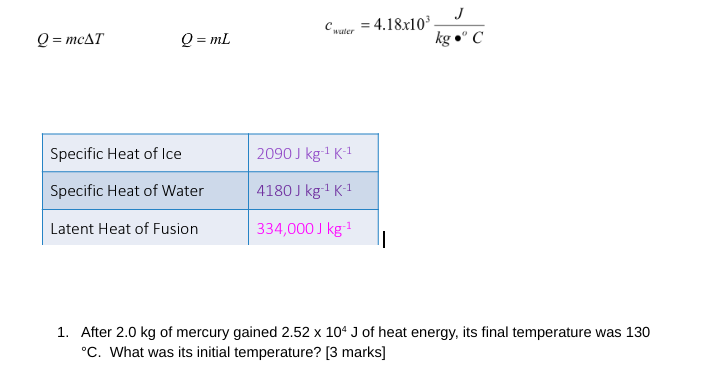

J Chapter = 4.18x103 Q = mcAT Q = ml kg ." C Specific Heat of Ice 2090 J kg-1 K-1 Specific Heat of Water 4180 J kg-1 K-1 Latent Heat of Fusion 334,000 J kg-1 1. After 2.0 kg of mercury gained 2.52 x 10* J of heat energy, its final temperature was 130 "C. What was its initial temperature? [3 marks]2. When doing an experiment to find the specific latent heat of fusion of liquid nitrogen, you find that 100kJ of heat must be removed to freeze 3.8kg of nitrogen at its freezing point. What value will you get for the specific latent heat of fusion of nitrogen? [2 marks] 3. A 0.20 kg piece of copper at 375.0"C is submerged in 0.50 kg of water at 18.0"C to be cooled quickly. Determine the final temperature of the copper and water. [4 marks] 4. How much heat does a refrigerator freezer have to remove from 0.10 kg of water at 22.0'C to make ice at -4.0"C? (*this requires you to think about what happens in relation to a cooling curve, there is more than one step to solve this problem). [6 marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


