Question: JBLEM #2 (30 points) Assuming that Eq. (10.45) is valid such that for the vapor we have B = B+w B where B& B1 are
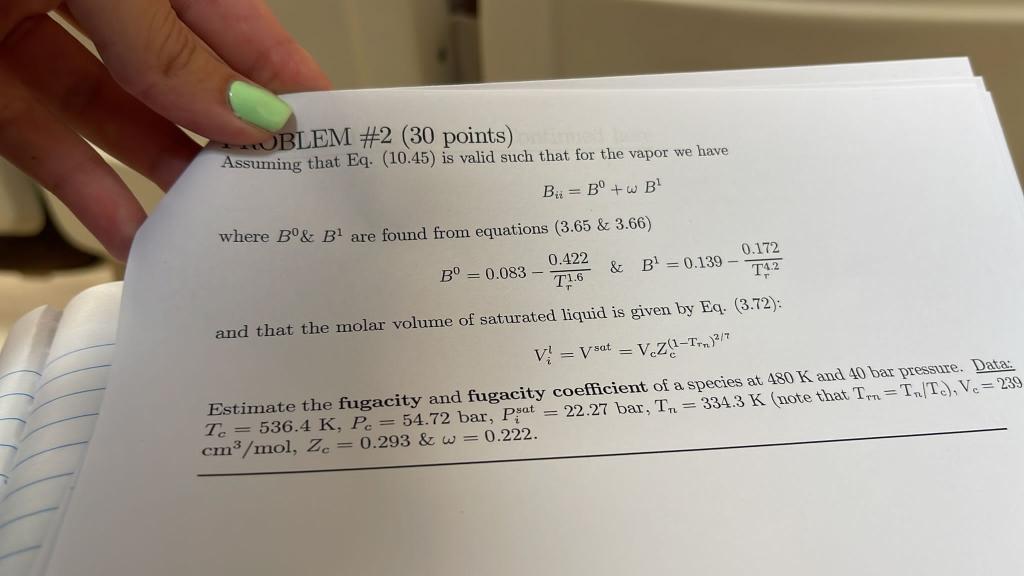
JBLEM #2 (30 points) Assuming that Eq. (10.45) is valid such that for the vapor we have B = B+w B where B& B1 are found from equations (3.65 & 3.66) 0.422 0.172 B = 0.083 - & Bl = 0.139 - T 1.6 14.2 and that the molar volume of saturated liquid is given by Eq. (3.72): V = V sat = V.Z(1-T.)2/7 Estimate the fugacity and fugacity coefficient of a species at 480 K and 40 bar pressure. Data: Te = 536.4 K, P = 54.72 bar, Psat = 22.27 bar, T = 3343 K note that Th=TT.). Ve= 239 cm3 /mol, ze = 0.293&w=0.222
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


