Question: Just answer the Initial Ideas section using the picture please! the reaction can be measured in the cell. Traditionally stopped-flow has lent itself to UV/Vis
Just answer the "Initial Ideas" section using the picture please!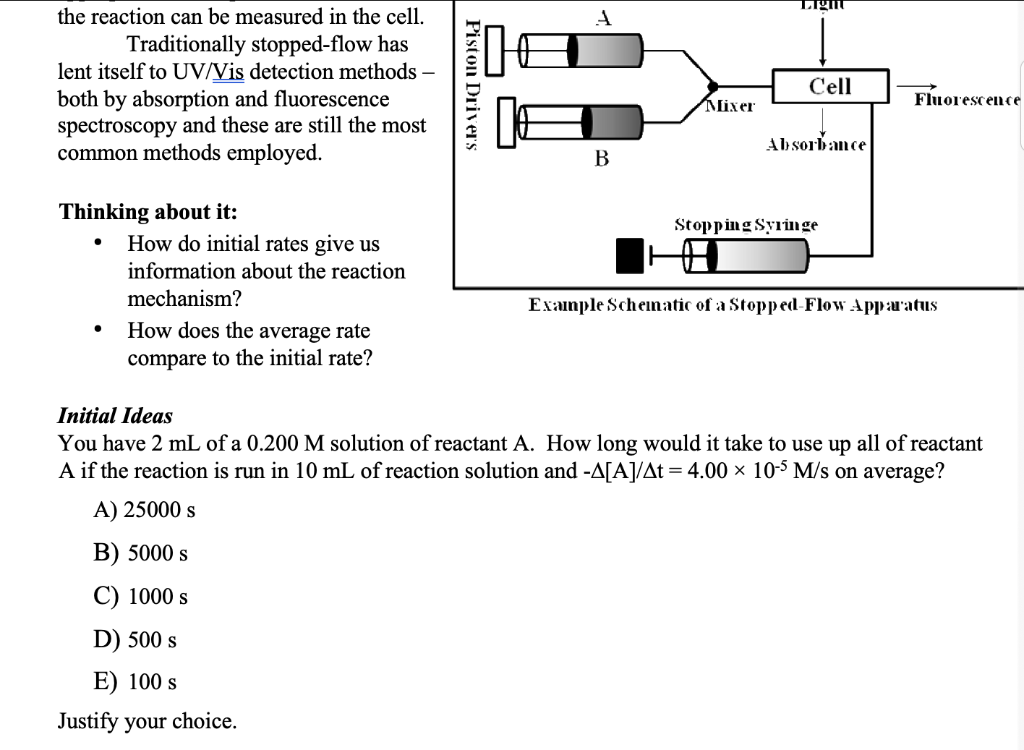
the reaction can be measured in the cell. Traditionally stopped-flow has lent itself to UV/Vis detection methods - both by absorption and fluorescence spectroscopy and these are still the most common methods employed. Thinking about it: - How do initial rates give us information about the reaction mechanism? Example Schematic of a Stopped-Flow Apprarus - How does the average rate compare to the initial rate? Initial Ideas You have 2mL of a 0.200M solution of reactant A. How long would it take to use up all of reactant A if the reaction is run in 10mL of reaction solution and [A]/t=4.00105M/s on average? A) 25000s B) 5000s C) 1000s D) 500s E) 100s Justify your choice
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


