Question: Just help with part b please. Please show how to complete the table. b) ( 6 pts) Experiments 4 through 9 contain sufficient information to
 Just help with part b please. Please show how to complete the table.
Just help with part b please. Please show how to complete the table.
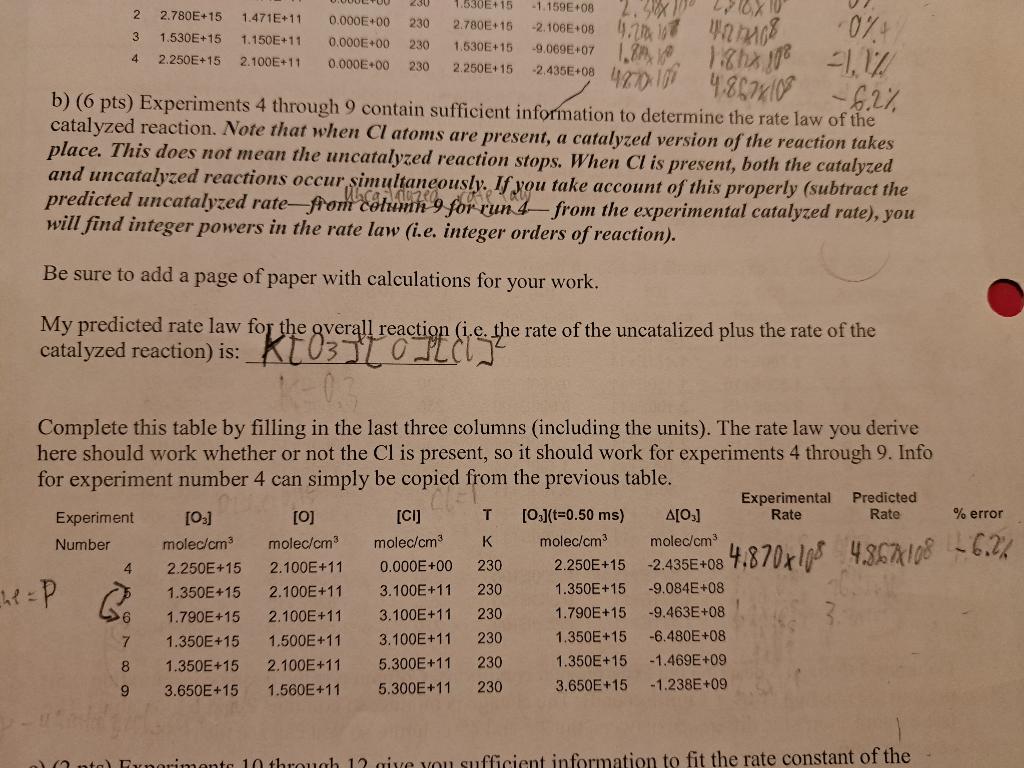
b) ( 6 pts) Experiments 4 through 9 contain sufficient information to determine the rate law of 6.2%, catalyzed reaction. Note that when Cl atoms are present, a catalyzed version of the reaction takes place. This does not mean the uncatalyzed reaction stops. and uncatalyzed reactions occur simultaneously. If you take accout is present, both the catalyzed predicted uncatalyzed rate for the properly (subtract the will find integer powers in the rate law (i.e. fromerimental catalyzed rate), you Be sure to add a page of paper with calculations for your work. My predicted rate law for the gverall reaction (i.e. the rate of the uncatalized plus the rate of the catalyzed reaction) is: K[03][0][(l]2 Complete this table by filling in the last three columns (including the units). The rate law you derive here should work whether or not the Cl is present, so it should work for experiments 4 through 9 . Info for experiment number 4 can simply be copied from the previous table. b) ( 6 pts) Experiments 4 through 9 contain sufficient information to determine the rate law of 6.2%, catalyzed reaction. Note that when Cl atoms are present, a catalyzed version of the reaction takes place. This does not mean the uncatalyzed reaction stops. and uncatalyzed reactions occur simultaneously. If you take accout is present, both the catalyzed predicted uncatalyzed rate for the properly (subtract the will find integer powers in the rate law (i.e. fromerimental catalyzed rate), you Be sure to add a page of paper with calculations for your work. My predicted rate law for the gverall reaction (i.e. the rate of the uncatalized plus the rate of the catalyzed reaction) is: K[03][0][(l]2 Complete this table by filling in the last three columns (including the units). The rate law you derive here should work whether or not the Cl is present, so it should work for experiments 4 through 9 . Info for experiment number 4 can simply be copied from the previous table
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


