Question: just need d an e solved needs to be done using fraction approach to unit conversion please. 2. (25pts) A gas is 50% Nitrogen (
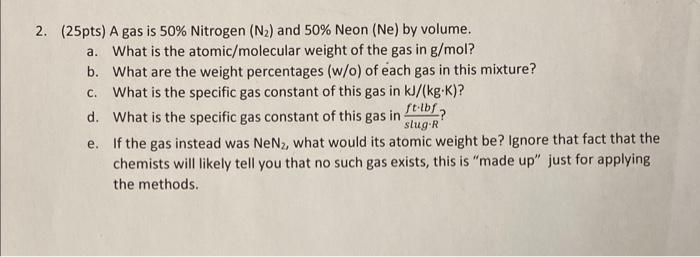
2. (25pts) A gas is 50% Nitrogen ( N2) and 50% Neon (Ne) by volume. a. What is the atomic/molecular weight of the gas in g/mol ? b. What are the weight percentages (w/o) of each gas in this mixture? c. What is the specific gas constant of this gas in kJ/(kgK) ? d. What is the specific gas constant of this gas in slugRftlbf ? e. If the gas instead was NeN2, what would its atomic weight be? Ignore that fact that the chemists will likely tell you that no such gas exists, this is "made up" just for applying the methods
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


